Analgesic Agent Analyzed in Tablet Form
Click HERE for Column Ordering Information.
There is a need for analysis of the Active Pharmaceutical Ingredient (API) in Ketorolac Tablet formulations in order to evaluate two different extraction protocols. The use of Ethanol as the extraction solvent was explored initially but DI Water was observed to produce superior extraction efficiency.
The chromatograms show how the compound can be retained and detected using either UV-based detection (Fig. A) or Mass Spectrometry (Fig. B). The conditions may be applied to the routine assay analyses of Ketorolac Tablet formulations.
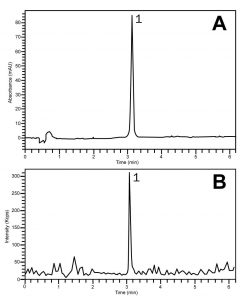
Peak:
Ketorolac 256.2 m/z [M+H]+
Method Conditions
Column: Cogent Phenyl Hydride™, 4µm, 1 00Å
Catalog No.: 69020-10P-2
Dimensions: 2.1 x 100 mm
Solvents:
—A: DI Water/ 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 20 |
| 5 | 80 |
| 10 | 80 |
| 11 | 20 |
Injection vol.: 1µL
Flow rate: 0.5 mL/minute
Detection:
—Fig. A: UV 320 nm
—Fig. B: ESI-POS- Mass Spec
Sample: Ground Ketorolac Tablet was extracted with DI Water (1 mg/mL powder concentration) and gravity filtered. Sample was then filtered through a 0.45µm Syringe filter for HPLC analysis.
Attachment
No 362 Ketorolac with HPLC and LCMS pdf 0.2 Mb Download File


