A Reproducible Method for Analysis of a Protease Inhibitor
Click HERE for Column Ordering Information.
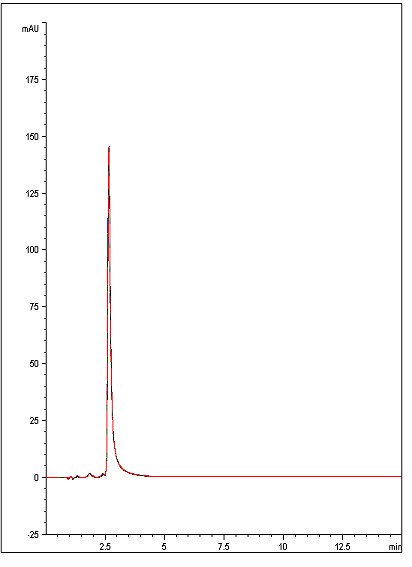
A rapid, sensitive, and Reproducible Method has been developed for this Antiretroviral Medication. The data below, (an overlay of 10 chromatograms ) illustrates how the compound can be adequately Retained and detected using this straightforward Method.
A Phenyl ring in the Column Stationary Phase provides strategic use of π-π Interaction with the Analyte making possible the use of a very simple, Mass Spec-friendly Mobile Phase with Formic Acid as an additive.
10 Injections of Ritonavir
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-10P
Dimensions: 4.6mm x 100mm
Mobile Phase: (65:35) Acetonitrile / DI Water with 0.1% Formic Acid
Injection vol.: 5μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: Ritonavir standard prepared as 1.0mg / mL Standard Solution in Mobile Phase
t0: 1.20 Minutes
K: 1.2
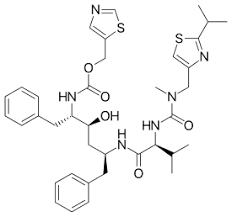
Notes: Ritonavir was initially developed as an independent Antiviral Agent but has been shown to possess advantageous properties in combination regimens with low-dose Ritonavir and other Protease Inhibitors. Currently, it is more commonly used as a booster of other Protease Inhibitors.