An Analysis of Vitamin C
Click HERE for Column Ordering Information.
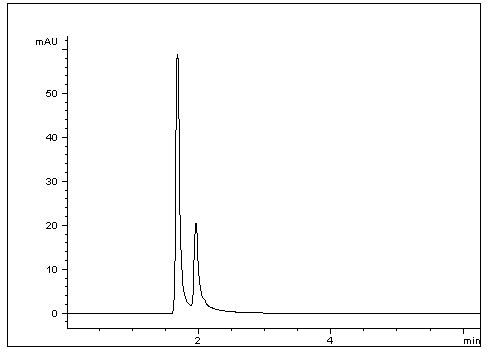
This simple Method shows good separation between two Enantiomers as well as Retention, considering these Acids both have a – 1.6 log p. This Method is Robust as results were verified with three different HPLCs and two separate Columns, all showing excellent Resolution of these two Acids.
Peaks:
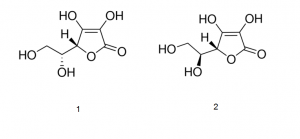
1. D- Isoascorbic acid
2. L- Ascorbic acid
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-10P
Dimensions: 4.6mm x 100mm
Mobile Phase: 98% Acetonitrile 2% DI Water / 0.1% Formic Acid
Flow Rate: 1.0mL / minute
Injection Volume: 1uL
Detection: UV 254nm
Injection vol.: 1μL
Sample Preparation: D- Isoascorbic Acid and L- Ascorbic Acid in 1.0 mg/mL in diluent of 50% Acetonitrile / 50% DI Water (v/v)
t0: 1.20 Minutes
K1: 0.39
K2: 0.62
α: 1.59
Note: Ascorbic acid exists as two enantiomers (mirror-image isomers), commonly denoted “l” (for “levo”) and “d” (for “dextro”). The l isomer is the one most often encountered and occurs naturally in many foods, and is one form of Vitamin C, an essential nutrient for many animals. Deficiency of Vitamin C causes scurvy. Vitamin C is used as a food additive and a dietary supplement for its antioxidant properties. The “d” form can be made via chemical synthesis but has no significant biological role.
Capacity Factor – Relative Retention k = (tR-t0)/t0
α= K2/K1