Separation of Ascorbic Acid, Riboflavin, Pyridoxine, & Thiamine
This LCMS compatible Method shows excellent Separation and Retention for all four analytes. If the analysis were done by Reversed Phase, LCMS incompatible ion pair agents would likely be required to get this type of separation.
Ascorbic Acid was found to have better Retention near neutral pH but Thiamine was retained too strongly under these conditions. Therefore a pH gradient was used in which the acidity of the Mobile Phase increases as well as the Water content. The Method is reliable and Robust with respect to analyte Retention and Peak shape, as the overlay of five consecutive runs in the Figure demonstrates.
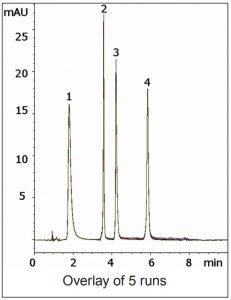
 Peaks:
Peaks:
1. Ascorbic Acid
2. Riboflavin
3. Pyridoxine
4. Thiamine
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 10mM Ammonium Formate / 0.05% Formic Acid (pH 3.5)
—B: 95% Acetonitrile / 5% 10mM Ammonium Formate (pH 6.5)
Gradient:
| Time (minutes) | %B |
| 0 | 100 |
| 1.5 | 100 |
| 4 | 30 |
| 6 | 30 |
| 7 | 100 |
Post Time: 3 minutes
Injection vol.: 1µL
Flow rate: 1.0mL / minute
Detection: UV @ 266nm
Sample Preparation: Mix of 300mg / L Ascorbic Acid, 5mg / L Riboflavin, 100mg / L Pyridoxine, 20mg / L Thiamine in 50% 10mM Ammonium Formate / 50% Acetonitrile diluent. Solution was filtered through 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.). Peak identities were confirmed by individual standards.
t0: 0.9 minutes
Note: The word “vitamin” was originally spelled “vitamine” when it was first coined by biochemist Casimir Funk. It was derived from the words “vital” and “amine” because it was believed at the time that all vitamins were chemical amines. The “e” was dropped from the word when it was discovered that this is not the case.
Attachment
No 164 pH Gradient to Separate Vitamins pdf 0.6 Mb Download File


