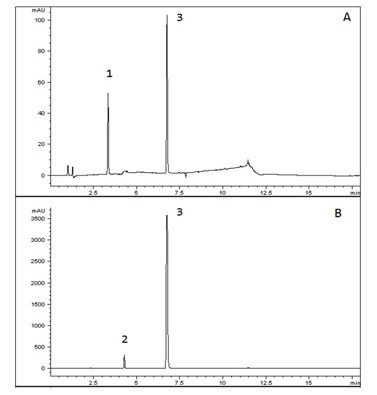
This AppNote demonstrates the USP Method for Amlodipine, Valsartan, and Hydrochlorothiazide Tablets working with the Cogent RP C18. As shown in the Chromatograms, the Peak efficiency is superb and USP Peak tailing guidelines are easily met with the Column. This demonstrates a great alternative Column for easy plug and play with this USP Method.
Method Conditions:
Column: Cogent RP C18™ 3um, 100A
Catalog No.: 68318-15P
Dimensions: 4.6 x 150mm
Mobile Phase:
—A. Acetonitrile/ DI Water / Phosphoric Acid (50:950:1)
—B. Acetonitrile/ DI Water / Phosphoric Acid (950:50:1)
Gradient:
| Time (minutes) | %B |
| 0 | 5 |
| 3 | 50 |
| 6 | 60 |
| 10 | 95 |
| 10.1 | 5 |
| 15 | 5 |
Injection Volume: 10uL
Flow Rate: 1.5ml / minute
Detection: UV @ 225 nm
Sample Preparation:
—A: Valsartan/Hydrochlorothiazide standards (0.16/0.025mg per mL)
—B: Valsartan/Amlopidine Sample (3.2/0.1mg per mL)
USP Method Page Information: USP43-NF38 – 279 USP42-NF37 – 274 USP41-NF36 1S – 8278 Most Recently Appeared In: Pharmacopeial Forum: Volume No. 43(2)
Note: Amlodipine, Valsartan, and Hydrochlorothiazide is a combination of medicines that is used to treat high blood pressure (hypertension). Amlodipine is a calcium channel blocker (CCB) that regulates the absorption of calcium into the cells of the heart and blood vessels. This causes the blood vessels to relax and increases the supply of blood and oxygen to the heart while reducing its workload. Valsartan is an angiotensin II receptor blocker (ARB) and works by blocking a substance in the body that causes the blood vessels to tighten. This lowers blood pressure and increases the supply of blood and oxygen to the heart. Hydrochlorothiazide is a thiazide diuretic (water pill). It reduces the amount of water in the body.
Attachment
N0.-381 Amlodipine, Valsartan, and Hydrochlorothiazide pdf 0.1 Mb Download File


