Separation demonstrated in real commercial formulation extracts
Use of the Cogent Bidentate C8™ Column allows for high resolution baseline separation of six specified impurities of Chlorpheniramine Maleate (Fig. A). Fig. B shows how the method can be applied to a real-world formulation, spiked with the N-oxide impurity to demonstrate resolution from the API peak.
Currently, there is no public official standard for Chlorpheniramine impurities analysis, hence this method supports quality testing for safety of products.
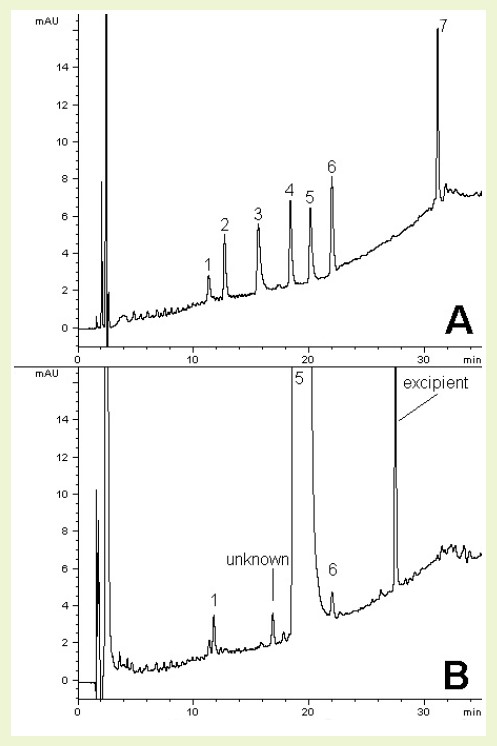
PEAKS:
1. Pheniramine
2. Chlorpheniramine related compound A
3. Chlorpheniramine related compound B
4. Chlorpheniramine related compound C
5. Chlorpheniramine
6. Chlorpheniramine N-oxide
7. Chlorpheniramine related compound D
Method Conditions
Column: Cogent Bidentate C8™, 4µm, 100Å
Catalog No.: 40008-15P
Dimensions: 4.6 x 150 mm
Solvents:
– A: 95% DI Water/ 5% Acetonitrile/ 0.05% TFA (v/v)
– B: Acetonitrile/ 0.05% TFA (v/v)
Gradient:
| Time (Minutes) | %B |
| 0 | 0 |
| 20 | 15 |
| 30 | 30 |
| 34 | 30 |
| 35 | 0 |
| 40 | 0 |
Injection vol.: 10µL
Flow rate: 1.0 mL/minute
Detection: UV 225 nm
Sample:
– Fig. A: 4.8 µg/mL each of USP Chlorpheniramine Maleate reference standard (RS), Pheniramine,
– Chlorpheniramine N-oxide, related compound (RC) A, B, C, and D.
– Fig. B: 4 mg strength Chlorpheniramine Maleate tablet extract (2.4 mg/mL) spiked at 0.1% level with
– Chlorpheniramine N-oxide Dihydrochloride RS solution.
Notes: Chlorpheniramine Maleate is an active pharmaceutical ingredient that is one of numerous over-the-counter antihistamine medicines used to treat allergic reactions such as hay fever and urticaria (hives). As with other first generation antihistamines, drowsiness can be a common side effect of the medication. This is due to their greater ability to cross the blood-brain barrier compared to second generation antihistamines.
No 368 Chlorpheniramine Maleate Organic Impurities.pdf 0.2 Mb Download File


