Polarity or Shape Recognition
An FDA requirement for Birth Control Product Analysis is the resolution of hormonal steroids ETES and ESTN. In this Method, Peaks ETES and ESTN are resolved with Polarity-based, Reversed Phase, ACN as the organic. When eluting with the shape Recognition in Methanol, ETES and ESTN are extremely well. However, in this test mix ETES now co-elutes with ESTD.
By mixing the Selectivity mechanisms, Polarity (ACN) and shape Recognition (MeOH), partial resolution was achieved on all compounds using the UDC Cholesterol Column in this Method. Scaling up to a 250 x 4.6mm Column resulted in baseline resolution of all Peaks.
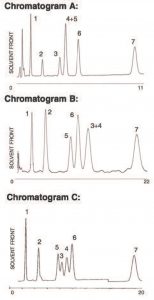
Peaks:
1. Prednisolone
2. Corticosterone
3. Estradiol
4. Ethinyl Estradiol
5. Estrone
6. Norgesterel
7. Progesterone
Method Conditions
Column: Cogent UDC-Cholesterol™, 4µm, 100Å
Catalog No.: 69069-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A. 60% Aqueous (0.1% Trifluoroacetic Acid TFA) / 40% Acetonitrile (ACN)
—B. 45% Aqueous (0.1% Trifluoroacetic Acid TFA) / 55% MeOH
—C. 56% Aqueous (0.1% Trifluoroacetic Acid TFA) / 24% MeOH and 20% ACN
Flow rate: 1mL / minute
Detection: UV @ 240nm
Notes: Using a single UDC Cholesterol Column with orthogonal selectivity, polarity in ACN and shape recognition in Methanol can lead to unique problem solving.
Attachments
No 18 Different Solvent Selectivity Mechanism with HPLC pdf Download File


