Isocratic Retention with LCMS
Gabapentin was retained successfully with a very symmetrical peak. Two different Mobile Phases were used and the advantage of the Mobile Phase used in Figure A is shown to be that Isopropanol present in the solvent A helps clean the Column which can be useful if biological samples containing lipids are used. Good retention of the drug was also achieved when solvent A was DI Water with 0.1% Formic Acid – Figure B and C.
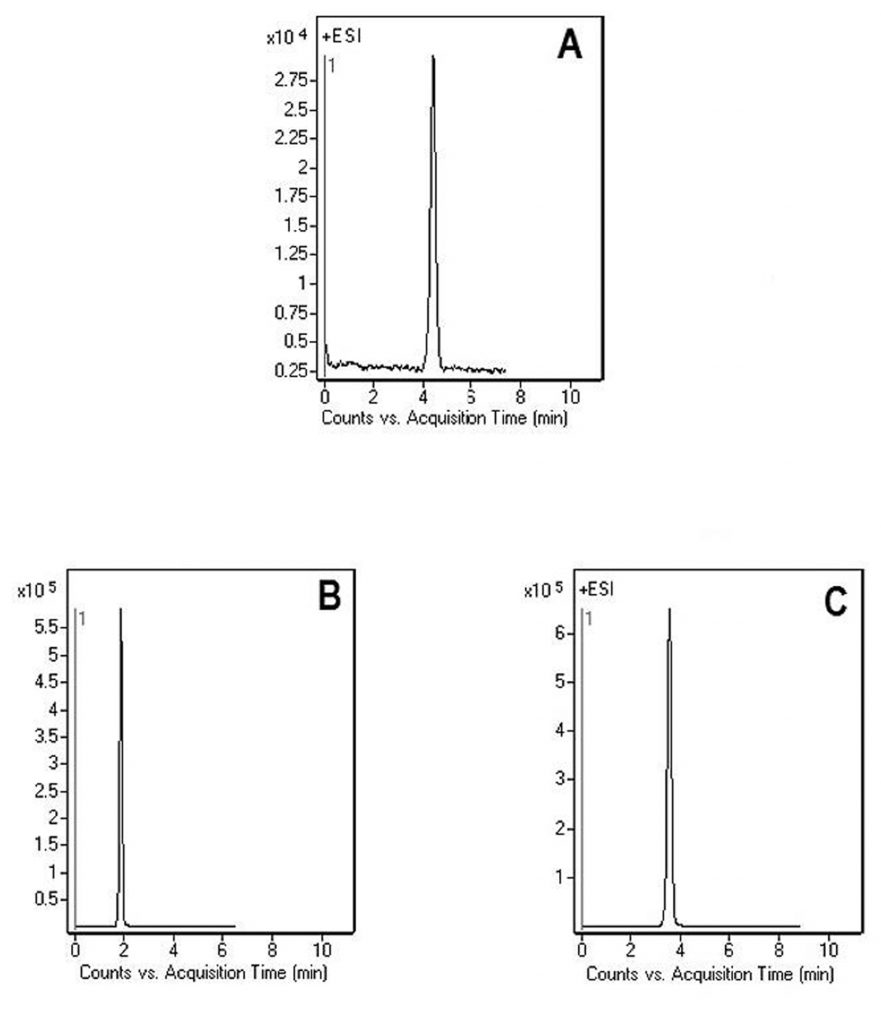
Peak:
Gabapentin 172 m/z (M+H)+
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase:
—Figure A:
—A: 50% Isopropanol / 50% DI Water/ 0.1% Formic Acid
—B: 97% Acetonitrile / 3% DI Water / 0.1% Formic Acid
—Figure B & C:
—A: DI Water + 0.1% Formic Acid
—B: 97% Acetonitrile / 3% DI Water / 0.1% Formic Acid
Post Time: 5 minutes
Total Time: 12 minutes
Flow rate: 0.4mL / minute
Detection: ESI — POS – Agilent 6210 MSD TOF Mass Spectrometer
Injection vol.: 1μL
Sample Preparation: Contents of 20 capsules were ground into a fine powder using a glass mortar and pestle. A portion equivalent to 100mg of Gabapentin was transferred into 100mL volume flask and sonicated for 15 minutes with 40mL of the 50% Solvent A and 50% Solvent B followed by 15 minutes on a shaker at 100 rpm. The flask was adjusted to volume and mixed well. The solution was filtered using a 0.45um Nylon Syringe Filter. 20uL of the filtered solution was added to 1mL of 50% Solvent A and 50% Solvent B mixture.
Note: Gabapentin is a Gamma-Aminobutyric Acid analog used for treatment of seizures in adults and children. It is structurally related to Neurotransmitter Gamma-Amminobutyric Acid (GABA). It freely crosses the blood-brain barrier and has been shown to increase GABA levels in the brain. Various analytical methods for therapeutic monitoring of Gabapentin in plasma or serum are described in the literature (GC, CE, HPLC), however all of these methods involve an extraction and derivatization step. It is well known that derivatization has drawbacks which include: the possibility of incomplete derivatization, additional interfering products, increased Method complexity, increased costs (additional reagents and increased preparation time). Gabapentin marketed as Neurontin™ has been approved for sale as a generic product.
Attachment
No 121 Gabapentin Drug Product Analysis by LCMS pdf 0.2 Mb Download File


