USP Method for Felodipine ER Tablets
This method presents the USP Felodipine Extended-Release Tablet assay using the Cogent RP C18. As shown in the chromatogram with a five injection overlay, the peak efficiency is superb and USP peak tailing guidelines are easily met with the Cogent RP C18™ column. This demonstrates a great alternative Column for easy plug and play or alternate for this USP Method.
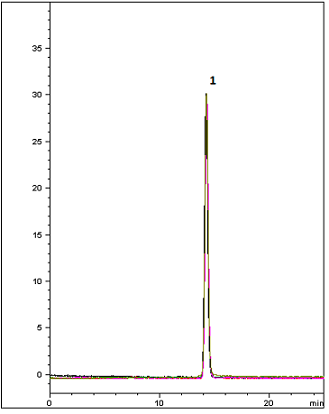
 Peak:
Peak:
Felodipine
Method Conditions:
Column: Cogent RP C18™, 5μm, 100Å
Catalog No.: 68518-15P
Dimensions: 4.6 x 150 mm
Buffer: 6.9 mg/mL of Monobasic Sodium Phosphate in Water. Adjust with 1 M Phosphoric Acid to a pH of 3.0 ± 0.05
Mobile Phase: Acetonitrile, Methanol, and Buffer (40:20:40)
Injection vol.: 40μL
Flow rate: 1.0 mL/minute
Detection: UV @ 362 nm
Sample: Standard solution: 0.02 mg/mL of USP Felodipine in Mobile phase
Most recently appeared in Pharmacopeial Forum: Volume No. 43(6) Page Information:
—USP43-NF38 – 1827
—USP42-NF37 – 1787
—USP41-NF36 – 1690
Note: Felodipine is a calcium channel blocker and acts primarily on vascular smooth muscle cells by stabilizing voltage-gated L-type calcium channels in their inactive conformation. Felodipine prevents myocyte contraction by binding to calcium-binding proteins, which exhibits competitive antagonism of the mineralcorticoid receptor. This event results into inhibiting the activity of calmodulin-dependent cyclic nucleotide phosphodiesterase and blocking calcium influx through voltage-gated T-type calcium channels. This reduces movement of calcium into the cells of the heart and blood vessels and increases supply of blood and oxygen to the heart.
Attachment:
No. 387 Felodipine Extended Release USP Method pdf 84.7 Kb Download File


