Separation of Prodrug from a Degradant in Forced Degradation of Valtrex® Tablets
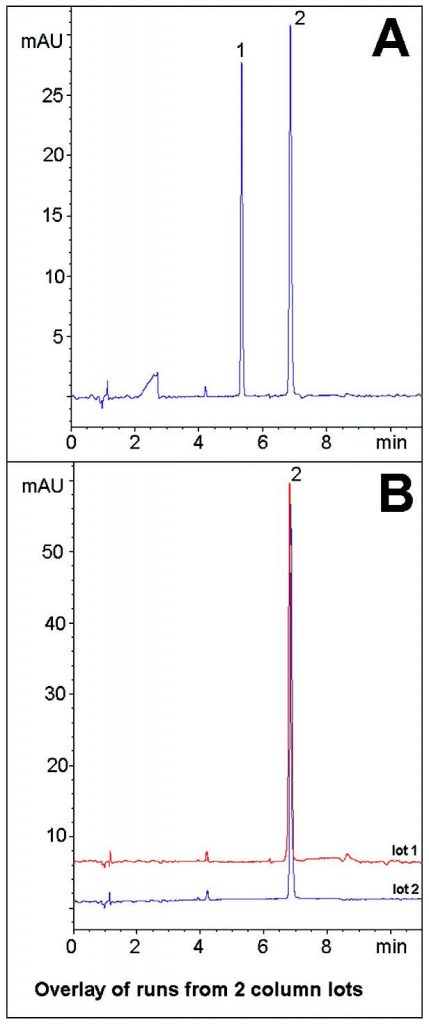
In this Application Note, the anti-viral Herpes drug Valaciclovir and its main acid degradant are well separated (Figure A). Valaciclovir is a prodrug and the degradant observed here is believed to be the active form, Acyclovir. Both compounds did not retain very strongly in Reversed Phase and the USP Method calls for a lengthy 40 minute Gradient with high water content for the Assay. The eight minute gradient provides sufficient Separation.
Data from two Column lots is shown below in the non-degraded extract (Figure B) to demonstrate the Method Reproducibility.
Peaks:
1. Degradant
2. Valaciclovir
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water with 0.1% Formic Acid (v/v)
—B: Acetonitrile with 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 95 |
| 1 | 95 |
| 6 | 40 |
| 7 | 40 |
| 8 | 95 |
Post Time: 3 minutes
Injection vol.: 1μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: Stock Solution: 1000mg strength Valtrex Tablet was ground and added to 100mL volumetric flask containing 50mL 50:50 DI Water / Acetonitrile diluent. The solution was sonicated 10 minutes, diluted to mark, and mixed. A portion was filtered through a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
—Figure A: Acid Degradation Extract: The Stock Solution was diluted 1:100 with 50:50 1N HCL / Acetonitrile mixture and heated at 85°C for 30 minutes.
—Figure B: Non-Degraded Extract: The Stock Solution was diluted 1:100 with 50:50 Acetonitrile / DI Water.
Attachment
No 254 Valaciclovir Analyzed with HPLC pdf 0.6 Mb Download File