Very Polar API Retained with Precision
This compound has two ionizable functional groups (an amine and a carboxylic acid), which makes it quite suitable for retention using this Method. A simple Gradient produced a very sharp Peak for this Analyte. These Method conditions can be applied to the routine Analysis of Baclofen Tablet formulations.
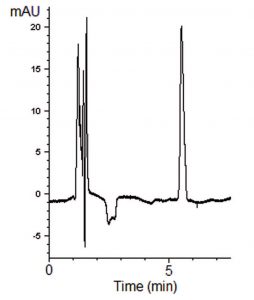
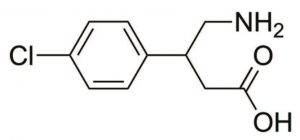
Peak:
Baclofen
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-15P-2
Dimensions: 2.1 x 150mm
Mobile Phase:
—A: DI Water with 0.1% Formic Acid (v/v)
—B: Acetonitrile with 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 95 |
| 5 | 30 |
| 6 | 30 |
| 7 | 95 |
Injection vol.: 1μL
Flow rate: 0.4mL / minute
Detection: UV @ 259nm
Sample Preparation: A Baclofen tablet (10mg strength) was ground with mortar and pestle, and 1.0mg of the ground powder was added to a beaker. Then 250μL diluent (66:4:30 DI Water / Glacial Acetic Acid / Methanol) was added and the solution was sonicated for 15 minutes. After gravity filtration, the filtrate was evaporated under vacuum and reconstituted in 250μL 90:10 Acetonitrile / DI Water with 0.1% Formic Acid (v/v).
Note: Baclofen is used in the treatment of drug and alcohol addiction. In this usage, this drug lessens the symptoms of withdrawal. Treating addiction by prescribing baclofen is considered an “off label” use of this drug. It was originally designed to treat epilepsy. There is a need for an analytical method to monitor use of this medication.
Attachment
No 360 Baclofen Analyzed with HPLC pdf 0.4 Mb


