Acetaminophen, Dextromethorphan, and Chlorpheniramine
Click HERE for Column Ordering Information.
This Method demonstrates the potential of the Cogent Phenyl Hydride 2.o™ Column for analysis of amine-containing analytes in a real formulation. Chlorpheniramine and Dextromethorphan can often be problematic in terms of peak tailing due to the tertiary amines, but this column and method produces excellent peak shapes for both compounds in this tablet formulation.
A high UV wavelength of 310 nm was chosen at the beginning of the gradient in order to reduce the Acetaminophen peak height, since this analyte is present in much higher concentration than the other two.
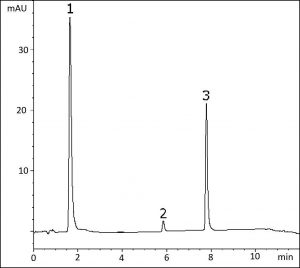
Peaks:
1. Acetaminophen
2. Chlorpheniramine
3. Dextromethorphan
Method Conditions
Column: Cogent Phenyl Hydride 2.o™, 2.2μm, 120Å
Catalog No.: 69220-05P-2
Dimensions: 2.1 x 50 mm
Mobile Phase:
—A: DI Water / 0.1% Trifluoroacetic Acid (TFA) (v/v)
—B: Acetonitrile / 0.1% Trifluoroacetic Acid (TFA) (v/v)
| Time (minutes) | %B |
| 0 | 5 |
| 2 | 5 |
| 9 | 80 |
| 11 | 80 |
| 12 | 5 |
Post Time: 5 minutes
Injection vol.: 1μL
Flow rate: 0.3mL / minute
Detection: UV @ 310nm (0-2 min), @ 265 nm (2-12 min)
t0: 0.7 minutes
Samples: Coricidin High Blood Pressure (HBP) Night® tablet containing 500mg Acetaminophen, 15mg Dextromethorphan HBr, and 2mg Chlorpheniramine maleate was ground and added to a 50mL volumetric flask. A portion of 50/50 solvent A/solvent B was added and the flask was sonicated for 10 minutes. Solution was then diluted to mark and mixed well. A portion was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.). This filtrate was used for injections and peak identities were confirmed with individual standards.
Attachment
No 324 Coricidin HBP Night Tablet pdf 0.2 Mb Download File


