A Robust Method for Analysis of a Hypercholesterolemia Medication
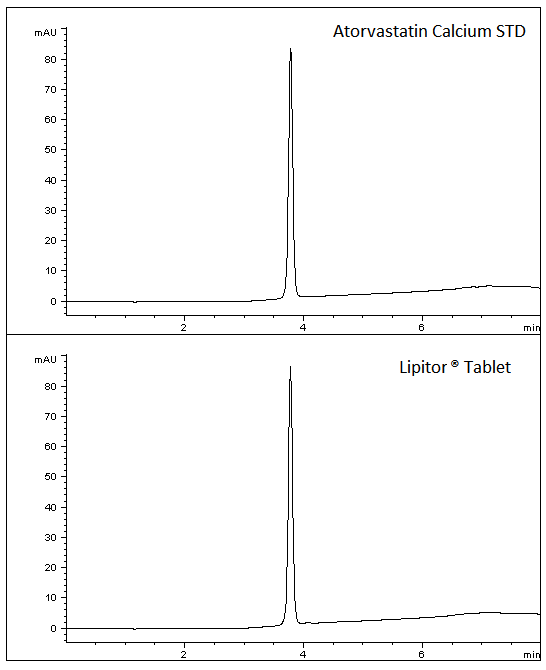
A robust and reproducible Method has been developed for this Cholesterol medication. A commercially available Drug Product was used as well as a reference Standard. The data below illustrates how the Standards and Drug Product share excellent peak shape using this easy Method.
 Peak
Peak
1. Top Chromatogram – Atorvastatin Calcium Standard
2. Bottom Chromatogram – Atorvastatin API from Tablets – Generic
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-10P
Dimensions: 4.6mm x 100mm
Mobile Phase:
—A: DI Water with 0.1% Formic Acid
—B: Acetonitrile with 0.1% Formic Acid
| Time (minutes) | %B |
| 0 | 50 |
| 1 | 50 |
| 5 | 85 |
| 6 | 85 |
| 7 | 50 |
| 8 | 50 |
Injection vol.: 2μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Diluent: 50:50 DI Water / Acetonitrile with 0.1% Formic Acid
Standard Preparation: Atorvastatin Calcium standard prepared as 0.1mg / mL standard solution in diluent.
Sample Preparation: 20mg strength tablet (Atorvastatin Calcium) was added to a 10mL volumetric flask with a portion of Diluent. The solution was sonicated 10 minutes and diluted to mark with Diluent. It was then filtered through a 0.45μm Nylon Syringe Filter (MicroSolv Technology Corp.). The filtrate was diluted to final concentration of 0.1mg / mL.
t0: 1.2 Minutes
K: 2.15
%RSD of 5 injections: ‹0.1%
Notes: Atorvastatin can treat high cholesterol and triglyceride levels. This may reduce the risk of angina, stroke, heart attack, and heart and blood vessel problems. Atorvastatin is a specific inhibitor of HMGCR (HMG-CoA reductase). HMGCR is the enzyme that catalyzes the conversion of HMG-CoA to Mevalonate, an early step in Cholesterol Biosynthesis. Atorvastatin is used in the treatment of Hypercholesterolemia. Marketed by Pfizer as Lipitor® this AppNote used a generic version.
Notes: Calculation for Capacity Factor – Relative Retention k = (tR-t0)/t0