Isocratic Separation of API from USP internal standard
The USP assay method for the active pharmaceutical ingredient (API) found in Nitrofurantoin Capsules, uses a Phosphate Buffer and is not compatible with Mass Spectrometry. In this AppNote, using the Cogent Bidentate C18 Column, Formic Acid is used as the Mobile Phase additive, as such, the method expands the capabilities of the assay to include LC-MS analyses. The USP system suitability for resolution between Nitrofurantoin and the internal standard Acetanilide is required to be not less than 3.0.
In this method, a Resolution of 8.2 was obtained, which exceeds this criterion. In addition, excellent repeatability is obtained for the analysis as shown in the figure below.
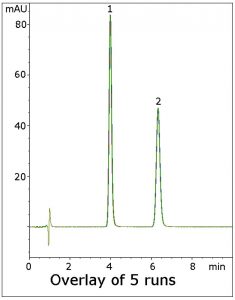
Peaks:
1. Nitrofurantoin (API)
2. Acetanilide
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75 mm
Mobile Phase: 85% DI Water / 15% Acetonitrile / 0.1% Formic Acid (v/v)
Injection vol.: 5μL
Flow rate: 1.0 mL/minute
Detection: UV @ 254 nm
Samples: 100mg strength Nitrofurantoin capsule contents were added to a 50 mL volumetric flask containing a portion of the mobile phase as diluent. The flask was sonicated for 10 minutes and diluted to mark. A portion was filtered with a 0.45μm Nylon Filter (MicroSolv Tech Corp.) 20μL of the filtrate and 100μL of a 1.0mg/mL Acetanilide solution were diluted with 880μL of the same diluent. Peak identities were confirmed by standards.
t0: 0.9 minutes
Note: Nitrofurantoin is an antibiotic primarily used to treat urinary tract infections and E. Coli. Once inside the bacterial cell wall, nitrofurantoin is reduced by flavoproteins to reactive intermediates which lead to its bactericidal effects. Various formulations are available in trade names such as Furadantin®, Macrobid®, and Furatin®.

Attachment
No. 188 Nitrofurantoin Capsule pdf 0.3 Mb Download File

