Chiral compound pairs are two non-superimposable mirror images of each other (see Fig. 1).
In their chemical structure, they must have one or more chiral centers (depicted by an up dash or down wedge). These chiral centers are usually but not always at a carbon.
Compounds that have more than one chiral center cannot be chiral if they have a plane of symmetry (i.e. they are superposable). These compounds are called meso (see Fig. 2). Compounds that have more than one chiral center are not chiral pairs if any of the chiral centers are the same between the two. In order to be chiral with respect to each other, they must have opposite chirality at all chiral centers in the compound. Compounds that have different chirality at some centers but not all are termed diastereomers. Compounds that differ at only one chiral center are called epimers. Chiral pairs are also termed enantiomers, R and S, (+) and (-), levorotatory and dextrorotatory, optical isomers, d and l, or D and L.
This wide variety of terminology comes from how the compounds are determined to be enantiomers. For example, the terms levorotatory and dextrorotatory come from which direction (clockwise or counter-clockwise) the compounds rotate the plane of polarized light. R and S come from naming conventions according to the Cahn–Ingold–Prelog rules. Enantiomers may behave differently in biological systems and therefore are of interest in pharmaceutical drug development. Only one enantiomer may exhibit efficacy while the other might be inactive or even harmful.
In 2009, 72% of the Top 200 Pharmaceutical drugs sales worldwide were chiral.
Fig. 1. Illustration of non-superimposable mirror images. The mirror image compound has its substituents in different directions than the original (e.g. if the blue and green substituents of the mirror image compound are aligned with the original, you will find that the red and yellow substituents will be switched compared to the original.)
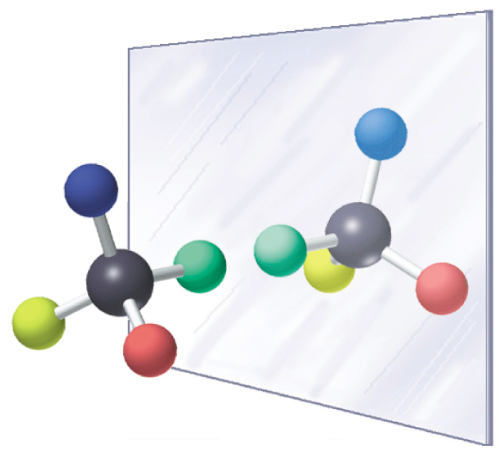
Fig. 2. Illustration of a meso compound. Although two chiral centers are present, the compound is not chiral because it has a plane of symmetry. Note that if one of the chiral centers is reversed, the compound will not have a plane of symmetry and therefore will be chiral.
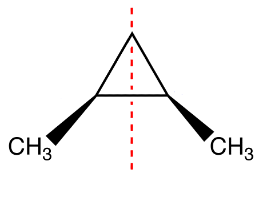
Table of Terms
| Term | Definition |
|---|---|
| chiral | of or relating to a molecule that is not superimposable on its mirror image |
| diastereomer | a stereoisomer of a compound having two or more chiral centers that is not a mirror image of another stereoisomer of the same compound |
| enantiomer | either of a pair of chemical compounds whose molecular structures have a non-superimposable mirror-image relationship to each other |
| meso | a compound that has two or more chiral centers but is not chiral due to a plane of symmetry |
| epimer | stereoisomers that differ in configuration of only one stereogenic center |
| racemic mixture | A 50:50 mixture of both enantiomers of a compound. The mixture will have no optical activity because enantiomers rotate the plane of polarized light in equal and opposite amounts, thereby cancelling each other out. |

