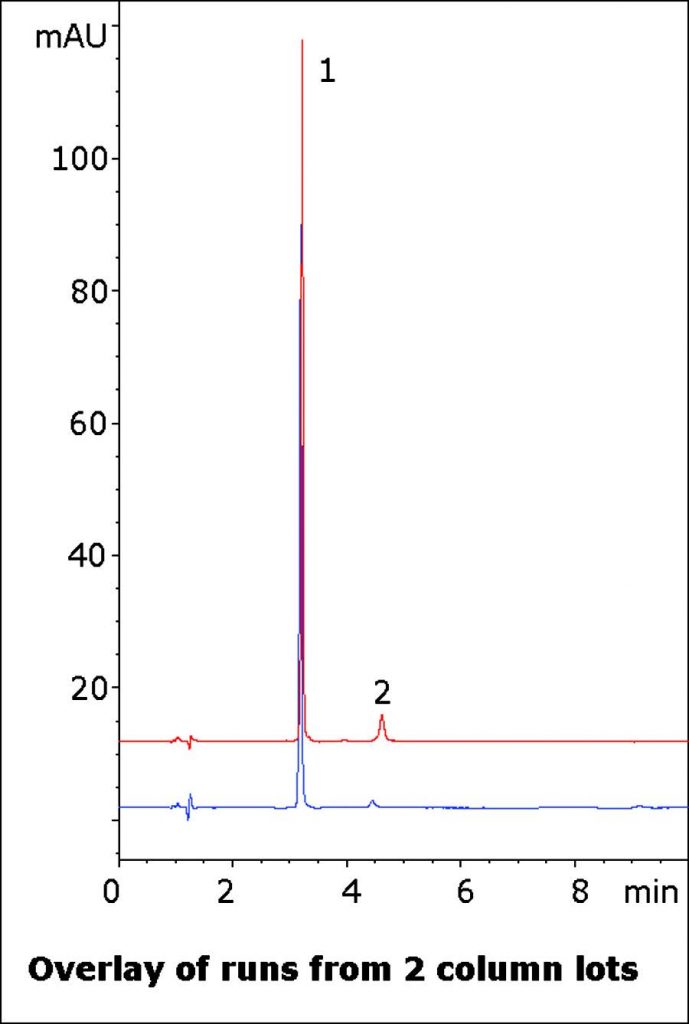
Polar API Separated from a Matrix Peak
Oxymetazoline can be difficult to obtain a good Peak Shape with conventional HPLC Methods, and the USP System Suitability requires that the Tailing Factor be not more than 2.0. Also, the USP Method calls for a Cation Exchange (L9) Column for the Assay. This Method produces excellent Peak Shape also shows Separation of the API from a matrix component or another ingredient in the formulation.
Two runs from different Column batches are overlaid in the Chromatogram to show the Method Robustness and Precision.
Peaks:
1. Oxymetazoline
2. Matrix component
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water with 0.1% Trifluoroacetic Acid (TFA)
—B: Acetonitrile with 0.1% Trifluoroacetic Acid (TFA)
Gradient:
| Time (minutes) | %B |
| 0 | 97 |
| 1 | 97 |
| 6 | 40 |
| 7 | 97 |
Post Time: 3 minutes
Injection vol.: 1μL
Flow rate: 1.0mL / minute
Detection: UV @ 280nm
Sample Preparation: Nasal spray solution containing 0.05% Oxymetazoline HCL was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.) and used for injections.
t0: 0.9 minutes
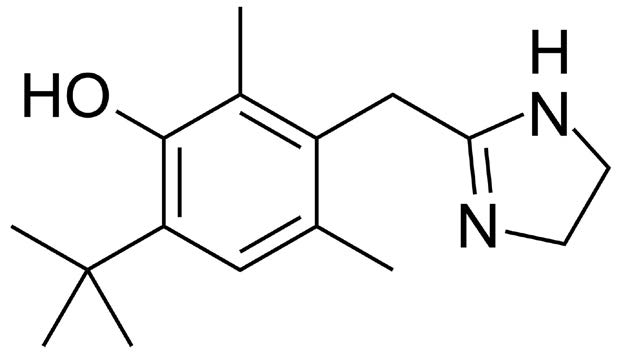
Note: Oxymetazoline is a decongestant that acts as a selective alpha-1 agonist and partial alpha-2 agonist. It is the active ingredient in many nasal spray solutions. It also has vasoconstriction properties and is therefore used in eye drop solutions as well.
Attachment
No 204 Oxymetazoline HCL Analyzed with HPLC pdf 0.4 Mb Download File