LCMS Compatible Method
Click HERE for Column Ordering Information.
The USP Assay Method for Cefprozil uses a phosphate-based Mobile Phase, which is not compatible with LCMS. In this Method, only Formic Acid is needed in the Mobile Phase. The Method meets the isomer resolution criterion of not less than 2.5 with an average calculated value of 4.4. The tailing factor was 1.0, which is within the USP acceptable range of 0.9–1.1.
Finally, the calculated efficiency was 5600 theoretical plates, which exceeds the requirement of not less than 2500. The excellent repeatability of the figure overlay illustrates the Method durability and fast Gradient equilibration.
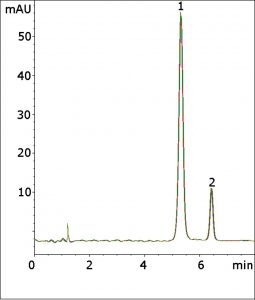
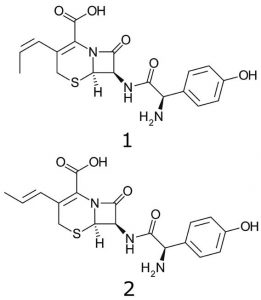
Peaks:
1. Cefprozil (Z-isomer)
2. Cefprozil (E-isomer)
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid
—B: Acetonitrile / 0.1% Formic Acid
Gradient:
| Time (minutes) | %B |
| 0 | 5 |
| 6 | 20 |
| 7 | 5 |
Temperature: 45˚C
Post Time: 1 minutes
Flow rate: 1.0mL / minute
Detection: UV @ 280nm
Injection vol.: 20μL
Sample Preparation:
—Stock Solution: 500 mg strength Cefprozil tablet was ground with a mortar and pestle. The ground tablet was added to a 100mL volumetric flask with 50mL 50:50 Solvent A / Solvent B diluent. The flask was sonicated 10 minutes, diluted to mark with the diluent, and filtered.
—Working Solution: A 100µL aliquot of the stock was diluted with 900µL of the diluent.
t0: 0.9 minutes
Note: Cefprozil is a cephalosporin antibiotic used to treat conditions such as bronchitis and infections of the ear, skin, throat, and sinuses.
Attachment
No 158 Cefprozil E & Z Isomers with HPLC pdf 0.3 Mb Download File


