Retention of Hydrophilic Analyte in a Drug Product
Click HERE for Column Ordering Information.
When Ethambutol is analyzed on many C18 Columns, it strongly interacts with residual Silanol groups in the Stationary Phase. These interactions normally cause excessive Retention and severe Peak broadening.
In this LCMS Method, Formic Acid is all that is required for the analysis of charged drugs like this one to produce excellent Peak Shapes. The Method can be used for Analysis of Ethambutol in Plasma Samples without the need for Pre-Column or Post-Column Derivatization.
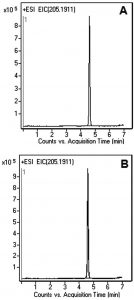
Peak:
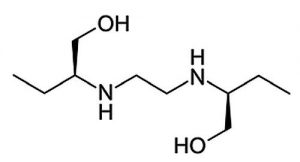
Ethambutol 205.1911 m/z (M +H)+
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: 97% Acetonitrile / 3% DI Water / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 95 |
| 1 | 95 |
| 3 | 20 |
| 5 | 20 |
| 6 | 95 |
Post Time: 3 minutes
Flow rate: 0.4mL / minute
Detection: ESI – POS – Agilent 6210 MSD TOF Mass Spectrometer
Injection vol.: 1μL
Sample Preparation: The contents of 20 commercially available capsules were ground using a mortar and pestle. A portion equivalent to 4–5mg of Ethambutol was transferred into a 50mL volumetric flask with 20mL of DI Water and was sonicated 15 minutes. Next, 20mL of Methanol was added and sonicated for 5 minutes. The flask was adjusted to volume with DI Water and mixed well. The solution was filtered using a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.). 5µL of the filtered solution was added to 10mL of a 50:50 Solvent A / Solvent B (v/v) mixture.
t0: 0.9 minutes
Note: Ethambutol is a medication used to treat tuberculosis. Trade names of the formulation include Myambutol® and Servambutol®.
Attachment
No 184 Ethambutol in Drug Products Analyzed with LCMS pdf 0.3 Mb Download File


