APAP Drug Substance Analysis with HPLC
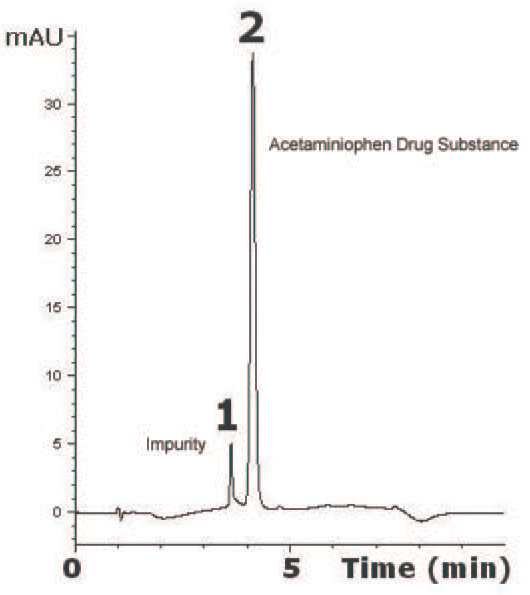
This Method has been developed for determination of Acetaminophen as a Drug Substance. The Separation was achieved on a C18 Column using Gradient Elution. The Method shown here has excellent Reproducibility of Acetaminophen for 5 consecutive Chromatograms shown had 0.03% RSD. This could be used for Quality Control of Acetaminophen.
Peaks:
1. Impurity
2. Acetaminophen
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Acetic Acid / 0.005% Trifluoroacetic Acid (TFA)
—B: 100% Acetonitrile / 0.1% Acetic Acid / 0.005% Trifluoroacetic Acid (TFA)
Gradient:
| Time (minutes) | %B |
| 0 | 0 |
| 1 | 0 |
| 4 | 30 |
| 6 | 30 |
| 6.01 | 0 |
| 10 | 0 |
Injection vol.: 2μL
Flow rate: 1mL / minute
Detection: UV @ 254nm
Sample Preparation: 1mg of the Compound was dissolved in 1mL of 50:50 Solvent A / Solvent B solution. Sample for Injection was diluted 1:15 with 100% Solvent A.
Notes: Acetaminophen (n-acetyl-p-aminophenol, APAP) is an non-steroidal anti-inflammatory drug (NSAID) which is widely used for the management of pain and fever. Safety concerns require analyzing the composition of the pharmaceutical formulations.
Attachment
No 68 Acetaminophen & Impurity Analyzed with HPLC pdf 0.1 Mb Download File