Forced Degradation Methods
Click HERE for Column Ordering Information.
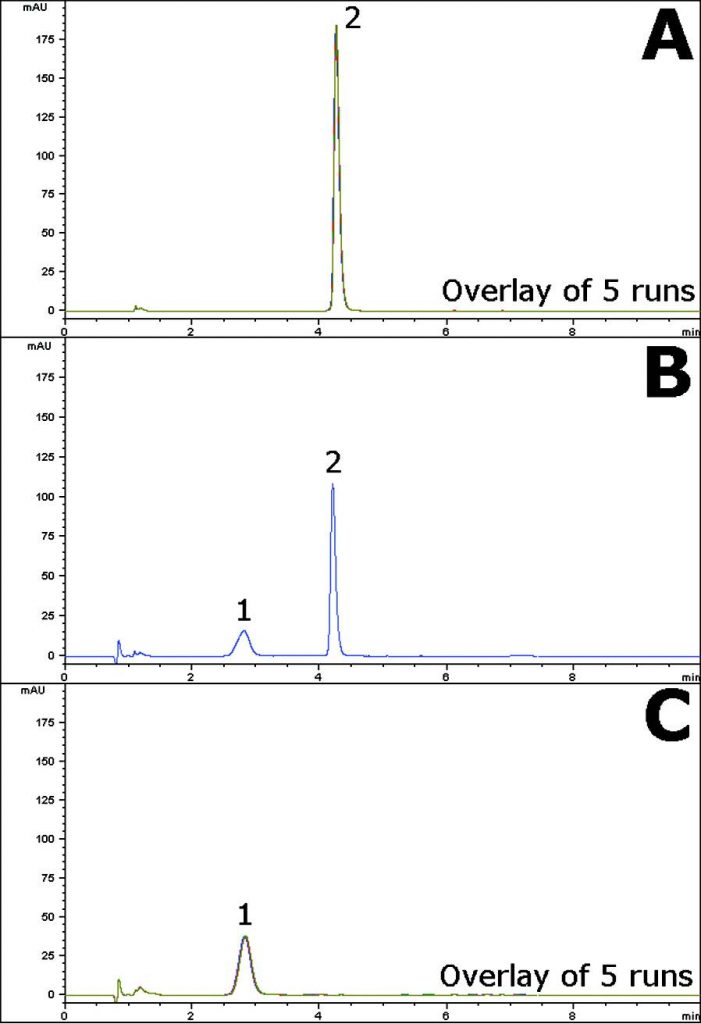
Forced degradation studies are useful for developing Stability Indicating Methods of Pharmaceuticals. In this Application Note Separation is obtained between the API and a Degradant formed under two different, strong acidic conditions. The non-degraded Sample produced a sharp, Symmetrical Peak for the API shown in Figure A.
In Figure B, strong acid conditions without heating produced a degradant while the API Peak was still present. In Figure C, acid degradation with heating completely converted the API into the degradant, as the API Peak was no longer observed.
Five Chromatograms are overlaid and presented below to show Method Robustness and Precision.
Peaks:
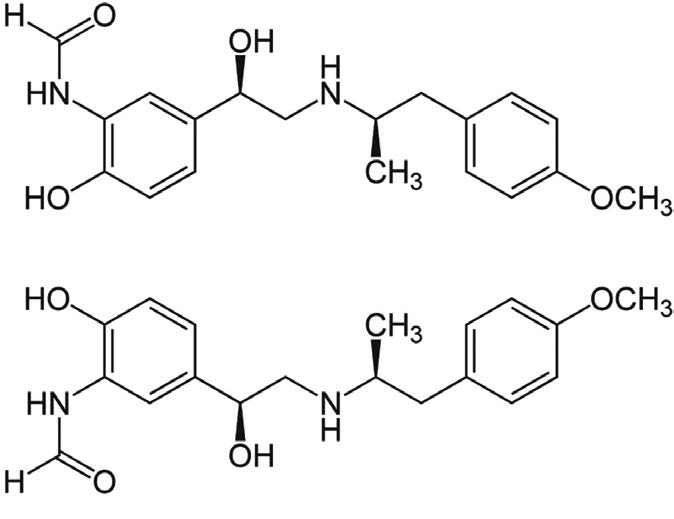
1. Degradant
2. Formoterol Showing Enantiomers
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Trifluoroacetic Acid (TFA) v/v
—B: Acetonitrile / 0.1% Trifluoroacetic Acid (TFA) v/v
Gradient:
| Time (minutes) | %B |
| 0 | 10 |
| 1 | 10 |
| 7 | 40 |
| 8 | 10 |
Post Time: 2 minutes
Injection vol.: 10μL
Flow rate: 1.0mL / minute
Detection: UV @ 282nm
Sample Preparation:
—Figure A: Non-degraded Sample: 0.1mg / mL Formoterol Fumarate in 50:50 Solvent A / Solvent B Diluent.
—Figure B: Acid degradation of the Sample, with no heating: 0.1mg / mL Formoterol Fumarate in 50:50 1N HCL / Acetonitrile Diluent.
—Figure C: Acid degradation of the Sample, with heating: 0.1mg / mL Formoterol Fumarate in 50:50 1N HCL / Acetonitrile Diluent after 30 minutes of heating at 85°C.
t0: 0.9 minutes
Note: Formoterol is used a long-acting ß2-antagonist used in treatment of conditions such as asthma and obstructive pulmonary disease. It is marketed under several trade names such as Foradil® and Oxeze®.
Attachment
No 178 Formoterol Fumarate pdf 0.4 Mb Download File