Retention with Good Peak Shape for this Polar Compound
Ciprofloxacin has several amine groups and therefore is susceptible to peak tailing in Reversed Phase methods. The USP assay method for Ciprofloxacin tablets uses Triethylamine as an Ion Pair Agent to reduce tailing and in addition, Phosphoric Acid is included in the Mobile Phase.
These conditions make the USP method incompatible with MS detection and therefore limit its applications. In this method, only Formic Acid is required in order to obtain a peak that meets the system suitability specification for the API tailing factor. This Method demonstrates how various Type-C™ application notes can be successfully adapted for LCMS.
The Peak Shape for this polar compound meets the System Suitability specification for the USP Tailing Factor.
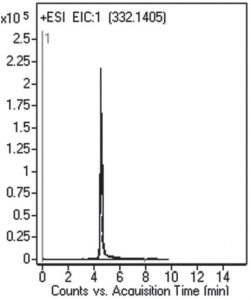
 Peak:
Peak:
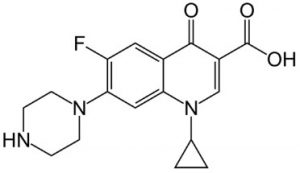
Ciprofloxacin 332.1405 m/z (M+H)+
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-15P-2
Dimensions: 2.1 x 150mm
Mobile Phase:
—A: 50% DI Water / 50% 2-Propanol / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 90 |
| 3 | 10 |
| 6 | 10 |
| 7 | 90 |
Post Time: 3 minutes
Injection vol.: 1µL
Flow rate: 0.4mL / minute
Detection: ESI – pos – Agilent 6210 MSD TOF Mass Spectrometer
Sample Preparation: A 250mg strength Ciprofloxacin tablet was ground and dissolved in 25mL of 50:50 Solvent A / Solvent B diluent. Solution was sonicated 10 minutes and filtered through 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.). Sample for injection was diluted 1:100 with the same diluent.
t0: 0.9 minutes
Note: Ciprofloxacin is a Fluoroquinolone antibiotic which acts by inhibition of DNA Gyrase and Topoisomerase IV. It was once used as a drug of last resort for bacterial infections, but accumulated resistance has made the drug less effective in recent years.
Attachment
No 182 Ciprofloxacin Analyzed with LCMS pdf 0.2 Mb Download File


