EDTA does not have a significant Chromophore, so to achieve UV Detection, in the Method shown below we used a pre-Column reaction of a Solution of Ferric Chloride with the Sample. The resulting EDTA/Fe3+ has significant UV Absorbance making this a very Sensitive Method.
Ethylenediaminetetraacetic acid is extremely difficult to analyze by itself however in its complexed form, it chromatographs well from matrices such as river sediment and other solutions.
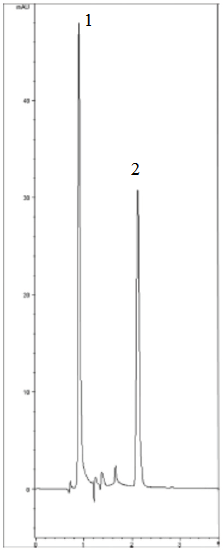
Peaks:
1. Water (solvent front)
2. EDTA Fe3+
Method Conditions:
Column: Cogent HPS C8™, 5μm, 120Å
Catalog No.: 75008-15P
Dimensions: 4.6 x 150mm
Mobile Phase: 98:2 DI H2O/ Acetonitrile with 0.1% Acetic Acid (pH 3.5/2gL Tetrabutylammonium Sulfate)
Temperature: 40°C
LOQ: 0.2μg / mL
Injection vol.: 20μL
Flow rate: 2mL / minute
Note: EDTA is a synthetic metal complexing reagent that is used in a wide variety of industrial applications. Used a preservative, it has very low biodegradability thus remains in the environment for long periods of time. Found in sewer water, freshwater and ground water, it re-solubilizes precipitated toxic metals back into solution where they can be ingested by plants and animals.
Attachment
A74. EDTA Analysis with HPLC pdf 8.7 Kb Download File


