Retention and Peak Shape for Highly Polar Compound
As a highly hydrophilic compound, Lisinopril is not well-suited to Reversed Phase Methods. The USP assay method for Lisinopril uses a highly Aqueous Mobile Phase (96% 2.76 g / L Monobasic Sodium Phosphate adjusted to pH 5.0 / 4% Acetonitrile) in Reversed Phase with an L7 Column.
The Peak efficiency was found to be significantly low when using the USP method. In this method, hydrophilic retention is readily achieved (see Figure) with a symmetric Peak shape. The analyte retention shows good repeatability, as shown in the five-run overlay.
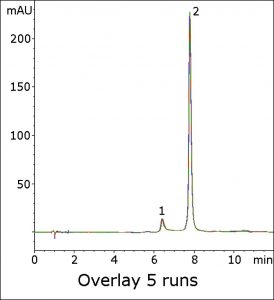
Peaks:
1. Impurity
2. Lisinopril
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 85 |
| 2 | 20 |
| 9 | 20 |
| 10 | 85 |
Post Time: 2 minutes
Flow rate: 1.0 mL / minute
Detection: UV @ 215nm
Injection vol.: 5μL
Sample Preparation:
—Stock Solution: 1mg / mL Lisinopril in 50% Solvent A / 50% Solvent B diluent
—Working Solution: Stock solution was diluted to 0.1 mg / mL with 50% Solvent A / 50% Solvent B diluent
t0: 0.9 minutes
Note: Lisinopril is an Angiotensin-Converting Enzyme (ACE) inhibitor that is used for treatment of cardiovascular conditions such as hypertension, congestive heart failure, and heart attacks.
Attachment
No 167 Lisinopril Analysis with HPLC pdf 0.3 Mb Download File


