Two methods for analysis of Promethazine Tablets address difficulties associated with HPLC for these formulations. The first one discussed is the Assay Method. Here a simple isocratic approach is used and a symmetrical peak shape is obtained for the API. The method meets system suitability criteria in terms of peak area precision, tailing factor, and plate count.
The impurity method; all of the known impurities could be resolved and quantitation of the specified impurity Promethazine Sulfoxide could be performed. The results showed it was below the safety threshold in this formulation.
Click Download File Below for Full Study Conditions.
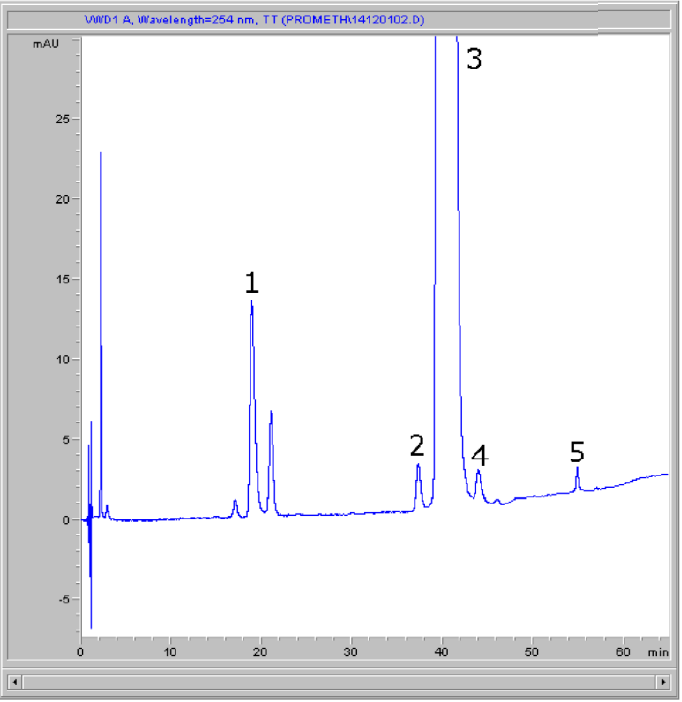
Peaks:
1. Promethazine Sulfoxide
2. N-Desmethyl Promethazine Derivate
3. Promethazine
4. Iso-Promethazine
5. Phenothiazine
Attachment
No 344 Promethazine Assay & Impurity Methods pdf 0.5 Mb Download File

