Separation from matrix peaks
Lansoprazole is separated from several Matrix Components in this simple assay method. The Mobile Phase is LC-MS compatible so the method could be applied to more complex samples such as plasma. Five runs are shown in the figure to illustrate the repeatability of the data.
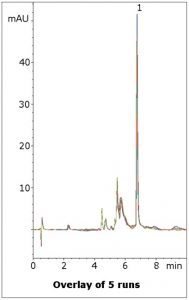
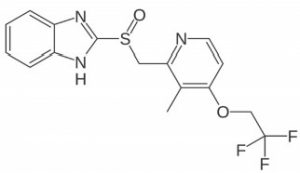 Peak:
Peak:
Lansoprazole
Method Conditions
Column: Cogent Bidentate C18™, 2.2µm, 120Å
Catalog No.: 40218-05P-2
Dimensions: 2.1 x 50 mm
Mobile Phase:
– A: DI Water / 0.1% Formic Acid (v/v)
– B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 10 |
| 1 | 10 |
| 6 | 70 |
| 7 | 10 |
Injection vol.: 0.2 μL
Flow rate: 0.4mL / minute
Detection: UV @ 285 nm
Sample Preparation: 15mg strength Lansoprazole capsule contents were ground and added to a 25mL volumetric flask. A portion of 50/50 Solvent A / Solvent B was added and the flask was sonicated 10 minutes. Then it was diluted to mark and mixed. A portion was filtered through a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.5 minutes
Note: Lansoprazole is a proton-pump inhibitor used for acid reducing effects. It is available over-the-counter by Novartis under the trade name Prevacid, as well as generic versions.
Attachment
No 273 Lansoprazole Capsule Analyzed with HPLC pdf 0.3 Mb Download File


