Analysis of Phenylephrine, a Highly Polar Compound
Phenylephrine was first studied under various Reversed Phase conditions using a C18 Stationary Phase, but even using a 95% Aqueous Mobile Phase a Retention factor of no more than 0.6 could be obtained. With such low retention, it may be difficult to Separate Phenylephrine from excipients or other APIs using this type of Column.
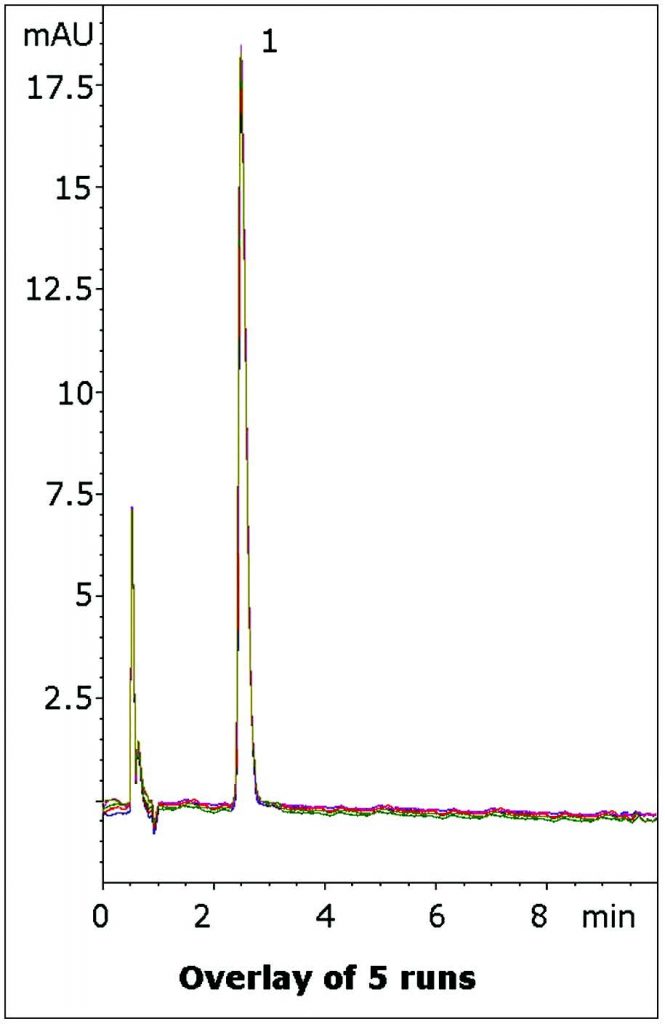
This Method produces a Retention factor of 3.7 with an excellent Peak Shape and could be suitable for routine assay of this API when in a Tablet Formulation.
Five Chromatograms are shown in the Figure to illustrate the repeatability of the data.
Method Conditions
Column: Cogent Diamond Hydride 2.o™, 2.2μm, 120Å
Catalog No.: 70200-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase: 5:95 A: DI Water with 0.1% Trifluoroacetic Acid (TFA) v/v, / B: Acetonitrile with 0.1% Trifluoroacetic Acid (TFA) v/v
Injection vol.: 0.2µL
Flow rate: 0.4mL / minute
Detection: UV @ 225nm
Sample Preparation: 10mg strength Phenylephrine HCL Tablet was ground and added to a 25mL volumetric flask. A portion of the Mobile Phase was added and the flask was sonicated 10 minutes. Then it was diluted to mark and mixed. A portion was filtered through a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.5 minutes
Note: Phenylephrine is a common decongestant used in many over-the-counter cold medications. Combination with other drugs is also prevalent in many formulations, so there is a need for a method for phenylephrine from other APIs.
Attachment
No 272 Phenylephrine HCL Tablet Analyzed with HPLC pdf 0.4 Mb Download File