Analysis of Imitrex® API from Tablet Formulations
This AppNote Method for Analysis of a challenging compound (Sumatriptan) has several advantages over other Methods. This Analysis can be performed using a higher concentration of organic solvent, which is much more suitable for electrospray MS Detection.
This AppNote provides robust and repeatable (%RSD 0.1 and lower) results.
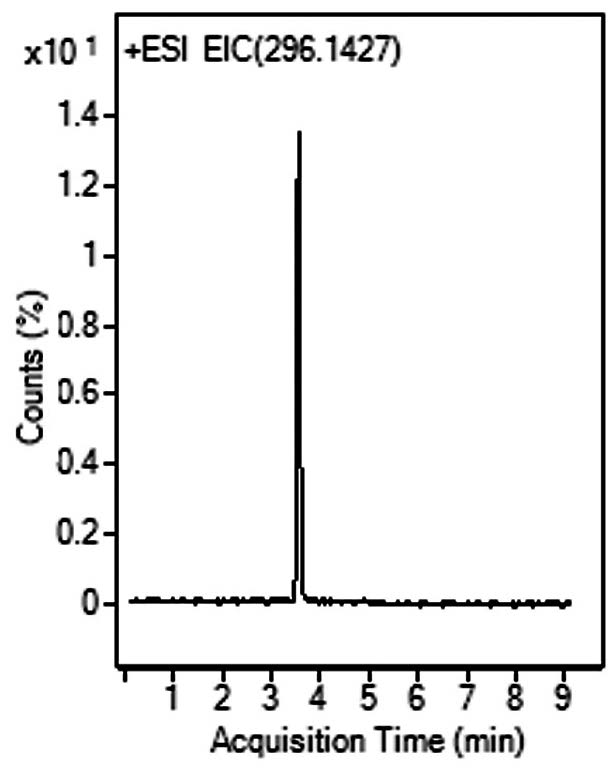
Peak:
Sumatriptan 296.1427 m/z [M+NH3]+
Method Conditions
Column: Cogent Diamond Hydride 2.o™, 2.2μm, 120Å
Catalog No.: 70200-05P-2
Dimensions: 2.1 x 50mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 90 |
| 4 | 30 |
| 6 | 30 |
| 7 | 90 |
Post time: 3 minutes
Injection vol.: 1µL
Flow rate: 0.4mL / minute
Detection: ESI – POS – Agilent 6210 MSD TOF Mass Spectrometer
Sample Preparation: Six 25mg strength tablets of Imitrex® were crushed and a portion containing 40mg of the API was weighed out. The powder was suspended in an Solvent A / Solvent B (1:1) Mixture, vortexed, and filtered through a 0.45μm Syringe Filter (MicroSolv Tech Corp.). A Sample for injection was diluted to final concentration of 0.0005 μg / mL.
t0: 0.9 minutes
Note: Migraine is a common disorder, with symptoms of unilateral headache accompanied by nausea and / or vomiting. Sumatriptan is an anti-migraine drug sold under the trade name Imitrex®. It belongs to a class of drugs called Selective Serotonin Receptor Agonists.
Attachment
No 265 Sumatriptan as an API Analyzed with LCMS pdf 0.2 Mb Download File