LC-MS Compatible Assay Method
Vardenafil in a tablet formulation can be readily assayed with this LC-MS compatible gradient method. The peak tailing factor was close to unity. The compound has several amine groups which can produce tailing with ordinary HPLC Columns that have a number of surface Silanols.
The MS-compatible Mobile Phase means that the method can be adapted to more complex samples such as plasma. Five runs are shown in the figure to illustrate the repeatability of the data.
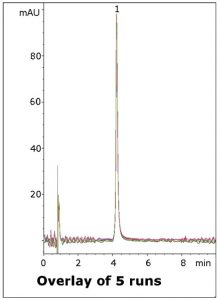
Peak:
Vardenafil
Method Conditions
Column: Cogent UDA™, 4µm, 100Å
Catalog No.: 40031-7.5P
Dimensions: 4.6 x 75 mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 90 |
| 1 | 90 |
| 6 | 40 |
| 7 | 90 |
Post Time: 3 minutes
Injection vol.: 2µL
Flow rate: 1.0 mL / minute
Detection: UV @ 210nm
Sample Preparation: 20mg strength Levitra® tablet was ground and added to a 25mL volumetric flask. A portion of 50 / 50 Solvent A / Solvent B diluent was added and the flask was sonicated 10 minutes. Then it was diluted to mark and mixed. A portion was filtered through a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.7 minutes
Note: Vardenafil is used to treat Erectile Dysfunction and acts by inhibition of Phosphodiesterase Type 5 (PDE5). It is sold under trade names Levitra® and Staxyn®.
Attachment
No 266 Vardenafil with HPLC pdf 0.4 Mb Download File


