“Green” Substitute for Acetonitrile in LCMS Method of 17 Amino Acids
Acetone has a high UV absorbance “cutoff” and therefore is generally unsuitable for HPLC with UV Detection. However, this is not a problem when using LCMS or Evaporative Light Scattering Detectors (ELSD) such as the Shimadzu Prominence or the Corona CAD. Acetone can be used to replace Acetonitrile as a Mobile Phase Component when using Cogent TYPE-C™ HPLC Columns and is a more environmentally friendly solvent [1] and it is easier to recover and reuse.
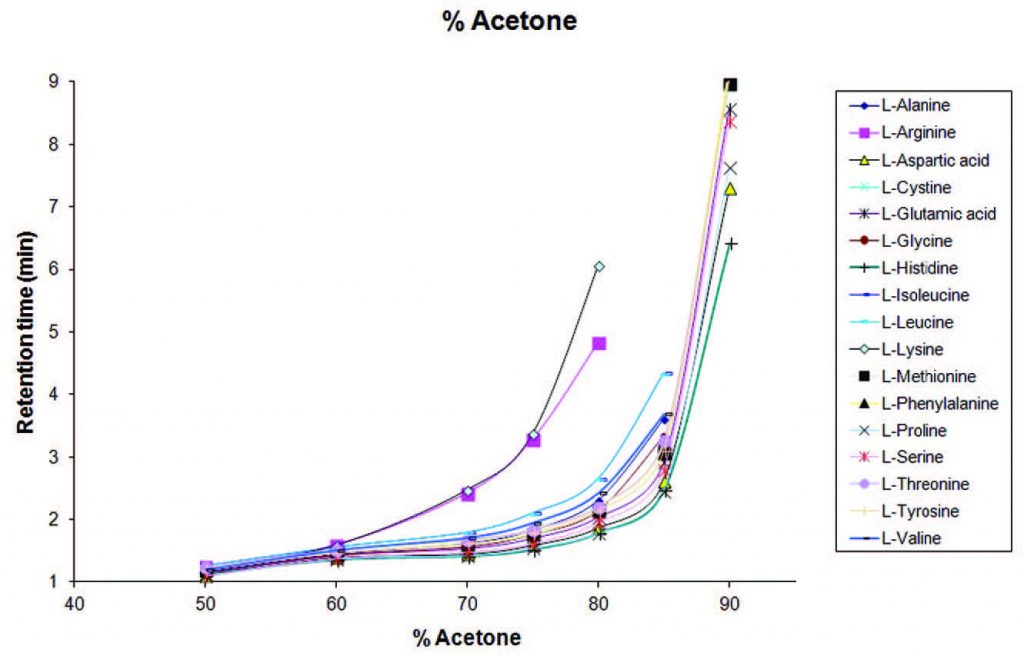
Interchangeability of Acetone and Acetonitrile: When amino acids are analyzed using Acetone as one of the Mobile Phase Components, the range for the onset of Retention is similar to what is obtained using Acetonitrile. Therefore Acetone and Acetonitrile can be used almost interchangeably for analysis of amino acids. It is important to note that while most compounds will retain and elute in similar fashion with either solvent, this cannot be said for every compound.
In the graph below, a Method was developed with an LCMS to determine the change in retention time with six different concentrations of Acetone as the Mobile Phase Component for 17 amino acids.
Method Conditions
Column: Cogent Diamond Hydride™, 4μm, 100Å
Catalog No.: 70000-15P-2
Dimensions: 2.1 x 150mm
Mobile Phase:
Step Gradient, 50–90% B in 10% Steps
—A: DI Water with 0.1% Formic Acid
—B: Acetone with 0.1% Formic Acid
Injection vol.: 1μL
Flow rate: 0.4mL / minute
Detection: ESI – POS – Agilent 6210 MSD TOF Mass Spectrometer
Sample Preparation: A solution of 17 amino acid standards (0.1mg / mL in DI Water) was prepared in DI Water. This solution was filtered through a disposable 0.45μm Syringe Filter (MicroSolv Tech Corp.). The sample for injection was diluted 1:100 with 50:50 Solvent A / Solvent B mixture.
Caution: Acetone and Amines can react when working with Acetone as an HPLC solvent it is important to note that primary amines could react with Acetone to form imines. However, to achieve this reaction the Mobile Phase would most likely need to be free of water and or acid as a catalyst. It is reported by the University of Liverpool that “Imines are formed when any primary amine reacts with an aldehyde or ketone under appropriate conditions. Imine formation requires an acid catalyst, otherwise the reaction is very slow. the acid is needed for the elimination of water.” Working with the above and other Methods in Aqueous Normal Phase HPLC, we have not seen the formation of imines occur.
[1] “Green” HPLC is Better and Easier Than You Think, by Joe Pesek and Bill Ciccone, Chromatography Today, November/December 2012 (Literature Reference).
Attachment
Acetone as a organic mobile phase component in LCMS 0.4 Mb Download File