Isocratic HPLC method for phenol in oral OTC products
This method shows how an assay of Phenol can be readily performed with a simple isocratic Mobile Phase. Phenol is the active ingredient in this common Over-The-Counter oral spray solution, which is intended for treatment of sore throat.
A number of inactive ingredients are present in the OTC solution as well but do not interfere with the API peak. Runs from two column lots are shown in the figure to demonstrate the stationary phase consistency. The Method can also be used in LCMS analyses as the Mobile Phase is MS-compatible.
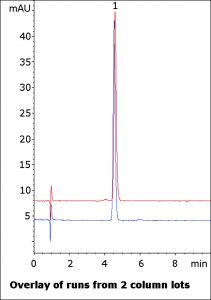
Peak:
Phenol
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase: 80% DI Water / 20% Acetonitrile / 0.1% Formic Acid (v/v)
Injection vol.: 2μL
Flow rate: 1.0mL / minute
Detection: UV @ 270nm
Sample Preparation: Chloraseptic® Solution containing 1.4% Phenol was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.). Solution was then diluted 1:100 with Mobile Phase diluent. Phenol peak identity was confirmed with a reference standard.
t0: 0.9 minutes
Note: Phenol is used in this solution as an oral anesthetic/analgesic. However, it also has applications as a starting material in many types of organic syntheses such as in plastics, pharmaceuticals, sunscreens, and cosmetics.
Attachment
Oral OTC Anesthetic Analyzed with HPLC pdf Download File


