A Simple Gradient with Good Separation
The official USP assay method for Docusate Sodium tablets calls for a Mobile Phase of 50% 7mM Ammonium Acetate / 50% Acetonitrile at 40°C. However, retention for the API and the System Suitability compound Methyl Paraben were found to be low using these conditions.
This Method not only exceeds the System Suitability resolution, but has both compounds adequately retained in order to allow for separation from other peaks that may be present in the sample. This Method is suitable for analysis of Docusate in a variety of matrices.
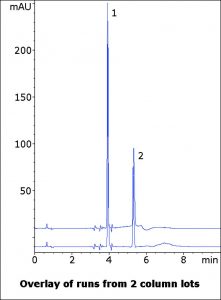
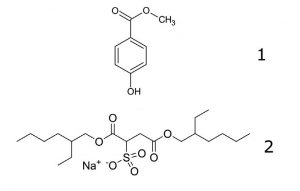 Peaks:
Peaks:
1. Methyl Paraben
2. Docusate Sodium
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 10 mM Ammonium Acetate
—B: 95% Acetonitrile / 5% Solvent A (v/v)
| Time (minutes) | %B |
| 0 | 20 |
| 1 | 20 |
| 6 | 80 |
| 7 | 20 |
Temperature: 40˚C
Post Time: 3 minutes
Injection vol.: 10μL
Flow rate: 1.0mL / minute
Detection: UV @ 210nm
Sample Preparation: 1.0mg / mL Docusate Sodium and 0.01mg / mL Methyl Paraben USP reference standards in diluent of 50:50 Solvent A / Solvent B. Peak identities were confirmed with individual standards.
t0: 0.9 minutes
Note: Docusate is used in many laxative formulations. It is also an emulsifying, wetting, and dispersing agent. It was one of the components in the oil dispersant Corexit® used to help clean the Deepwater Horizon oil spill of 2010.
Attachment
No 213 Docusate Sodium Analyzed with HPLC pdf 0.3 Mb Download File


