Improved Specificity Compared to USP Assay Method
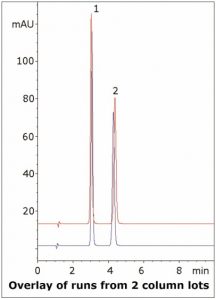
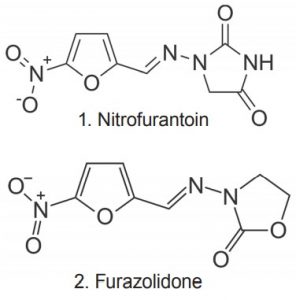
The USP Assay Method for Furazolidone is performed by UV Spectrophotometry. This HPLC Method provides more Robustness and Specificity for the analysis. Separation of Furazolidone from the structurally similar compound Nitrofurantoin is shown in the figure.
Nitrofurantoin can be used as an internal standard to obtain more robust Quantitation. Furthermore, the ability of this Method to distinguish amongst similar compounds demonstrates how it is less prone to interference from impurities or degradants. Two Chromatograms are overlayed to present the precision of the Method using two different Columns made from different lots.
 Peaks:
Peaks:
1. Nitrofurantoin (Internal Standard)
2. Furazolidone
Method Conditions
Column: Cogent Bidentate C8™, 4µm, 100Å
Catalog No.: 40008-75P
Dimensions: 4.6 x 75mm
Mobile Phase: 80% DI Water / 20% Acetonitrile / 0.1% Formic Acid (v/v)
Injection vol.: 1µL
Flow rate: 1.0mL / minute
Detection: UV @ 367nm
Sample Preparation: 1mg Furazolidone and 1mg Nitrofurantoin USP reference standards were dissolved in 1mL of the Mobile Phase. The solution was then diluted 1:10 with the same diluent. Peak identities were confirmed with individual standards.
t0: 0.9 minutes
Note: Furazolidone is an antibacterial Nitrofuran. It is used in both human and veterinary medicine. It is available under the trade name Furoxone®.
Attachment
No 205 Furazolidone Analyzed with HPLC pdf 0.6 Mb Download File


