Extremely Precise, Assay Method
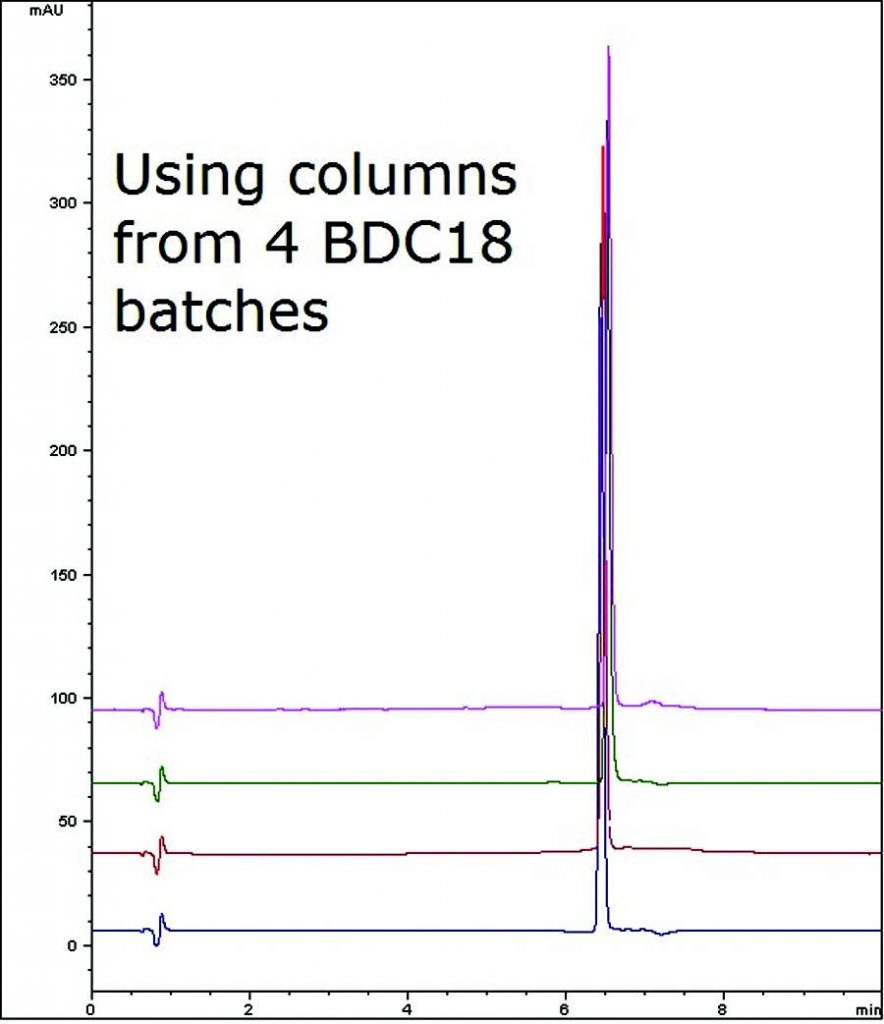
This Assay Method for a common Ibuprofen formulation demonstrates Reproducibility and Robustness as the Figure below shows an overlay of four Chromatograms with different Columns lots (%RSD < 1).
An important aspect of Column Selection for a Method is that the Retention behavior is consistent across numerous manufacturing batches. This is especially crucial once a Method has been validated and is in routine use.
Peak:
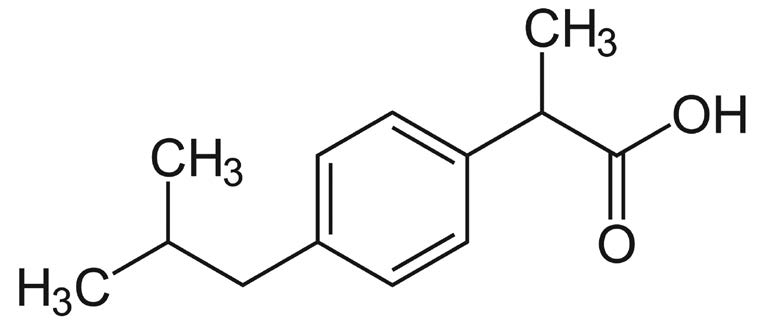
Ibuprofen
Method Conditions
Column: Cogent Bidentate C18™, (BDC18), 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water with 0.1% Formic Acid
—B: Acetonitrile with 0.1% Formic Acid
Gradient:
| Time (minutes) | %B |
| 0 | 30 |
| 2 | 30 |
| 6 | 70 |
| 7 | 30 |
Post Time: 3 minutes
Injection vol.: 10μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: 200mg strength Advil® tablet was ground and added to a 50mL volumetric flask with a diluent of 1:1 Solvent A / Solvent B. It was sonicated 10 minutes and diluted to mark. Then a portion was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Ibuprofen is a nonsteroidal anti inflammatory drug (NSAID) commonly used for its analgesic effects. It is marketed under a variety of trade names such as Advil and Motrin®. In addition, it is often included in combination formulations as well.
Attachment
No 195 Ibuprofen Analyzed with HPLC pdf 0.3 Mb Download File