Isocratic Method for Prednisone Tablets
This Method uses the Mobile Phase specified in the USP Method for Assay of Prednisone tablets. The resolution between the API and the Acetanilide Internal Standard meets the system suitability.
Resolution was found to be superior compared to standard C18 HPLC Columns. In addition, this Method also produces excellent precision under these conditions, as shown in the figure overlay.
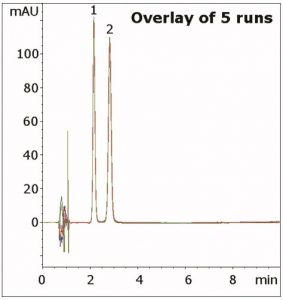
 Peaks:
Peaks:
1. Acetanilide (Internal Standard)
2. Prednisone (API)
Method Conditions
Column: Cogent Bidentate C8™, 4µm, 100Å
Catalog No.: 40008-75P
Dimensions: 4.6 x 75mm
Mobile Phase: 68.8% DI Water / 25.0% Tetrahydrofuran (THF) / 6.2% Methanol (v/v)
Injection vol.: 10µL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: 10mg strength Prednisone tablet was ground and added to a 25mL volumetric flask. It was diluted to mark with Methanol and sonicated 10 minutes. It was then filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Technology Corp.). 110µL of the filtrate and 90µL of a 0.1mg / mL Acetanilide solution were combined and diluted with 800µL Methanol. Peaks were confirmed by individual standards.
t0: 0.8 minutes
Note: Prednisone is used to treat symptoms due to low corticosteroid levels. This encompasses a wide variety of applications such as for Arthritis, severe allergic reactions, Multiple Sclerosis, and Lupus. It is marketed under a variety of trade names, such as Deltasone®, Meticorten®, and Orasone®.
Attachment
No 189 Prednisone Analyzed with HPLC pdf 0.5 Mb Download File


