API Separation from Impurity without Ion-Pair Reagents
Click HERE for Column Ordering Information.
The USP Method for Quinine Sulfate requires a Resolution of not less than 1.2 from its main impurity, Dihydroquinine. In the USP Method, the ion pair agents Methanesulfonic Acid and Diethylamine are used in the Mobile Phase. Ion pair agents are often needed to reduce peak tailing of basic analytes such as Quinine when conventional HPLC Columns are used.
In this Method, Quinine is separated from its main impurity with a Resolution of 2.6 using only 0.1% Trifluoroacetic Acid in the Mobile Phase.
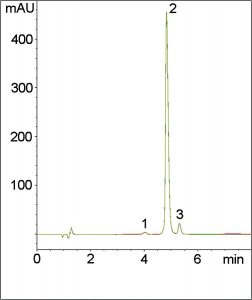
Peaks:
1. Minor impurity
2. Quinine (API)
3. Dihydroquinine (Main impurity)
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Trifluoroacetic Acid (TFA)
—B: Acetonitrile / 0.1% Trifluoroacetic Acid (TFA)
Gradient:
| Time (minutes) | %B |
| 0 | 10 |
| 6 | 30 |
| 7 | 10 |
Temperature: 40˚C
Post Time: 1 minute
Injection vol.: 10μL
Flow rate: 1.0mL / minute
Detection: UV @ 235nm
Sample Preparation:
—Stock Solution: 1.0mg Quinine (90% by label claim) was dissolved in 1mL of 50:50 Solvent A / Solvent B mixture.
—Working Solution: A 100μL aliquot of the stock was diluted with 900μL of 50:50 Solvent A / Solvent B mixture.
t0: 0.9 minutes
Note: Quinine is an alkaloid found naturally in the bark of Cinchona trees. It has been known for hundreds of years as an Antimalarial agent and a muscle relaxant. The Quechua inhabitants of Peru would grind the bark of Cinchona trees and mix it with sweetened water to reduce shivering in cold temperatures. Jesuits in Peru brought the bark to Europe where it was widely used to treat Malaria and became known as “Jesuit’s bark.”
Attachment
No 154 Quinine Sulfate and Impurity Analysis with HPLC pdf 0.3 Mb Download File


