Improved Gradient Method with Faster Equilibration
The USP Assay Method for Valsartan in combination with Hydrochlorothiazide features a 27 minute gradient with a 13 minute re-equilibration for a total run time of 40 minutes. In this method, the run time was a quarter of the USP method, and the Column equilibrates much faster when gradients are used. This demonstrates a substantial time and solvent savings for the analytical laboratory.
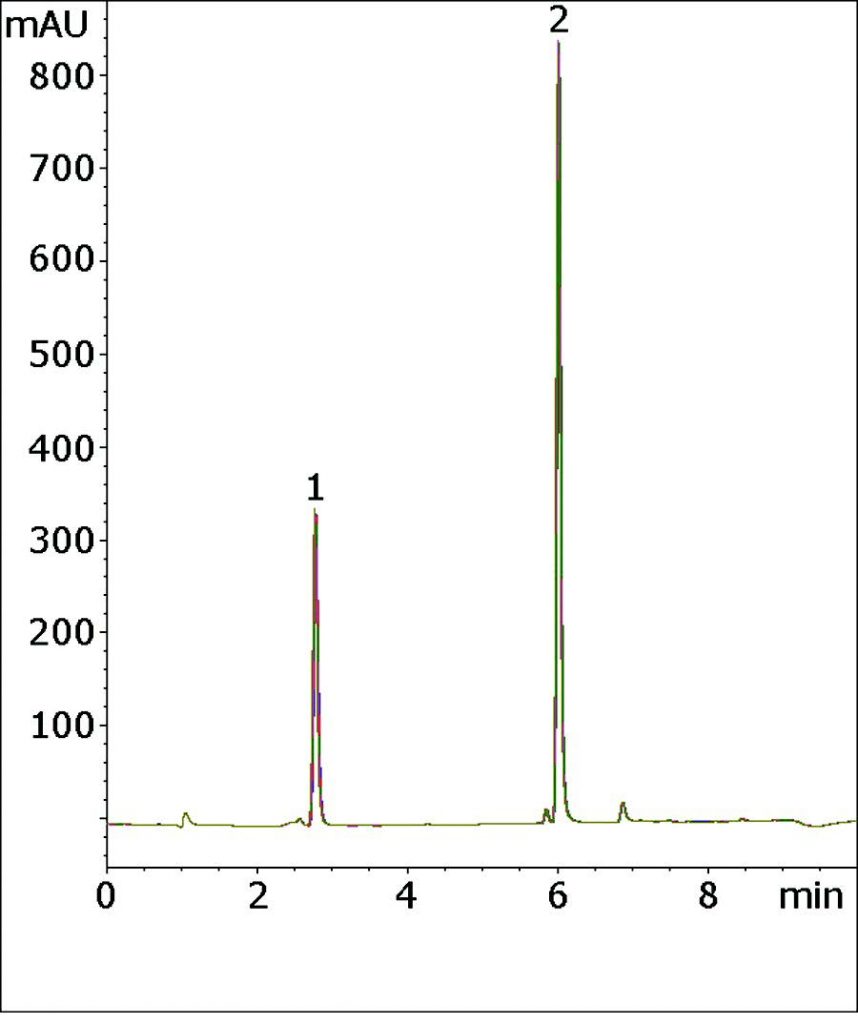
Five Chromatograms are overlaid below which shows the Robustness and Precision of this Method.
Peaks:
1. Hydrochlorothiazide (HCT)
2. Valsartan
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-75P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Trifluoroacetic Acid (TFA)
—B: Acetonitrile / 0.1% Trifluoroacetic Acid (TFA)
Gradient:
| Time (minutes) | %B |
| 0 | 10 |
| 8 | 90 |
| 9 | 10 |
Post Time: 1 minute
Injection vol.: 10μL
Flow rate: 1.0ml / minute
Detection: UV @ 265nm
Sample Preparation:
—Stock Solution: A Diovan® HCT brand tablet containing 160mg Valsartan and 25 mg Hydrochlorothiazide was ground and added to a 50 mL volumetric flask. The flask was diluted to mark with 50:50 Solvent A / Solvent B mixture and sonicated. A portion was then filtered with a 0.45μm Nylon Syringe Filter AQ™ Brand (MicroSolv Tech Corp.).
––Working Solution: 100μL of the stock solution was diluted with 900μL of a 50:50 Solvent A / Solvent B mixture.
t0: 1 minute
| Comparison of This Method and the USP Method | ||
| Bidentate C18 | Ordinary C18 | |
| Total Run Time | 10 Minutes | 40 Minutes |
| Column Volume Equilibration | 1 Column | 10 Columns |
| Solvent Usage per Run | 10ml | 40ml |
Attachment
No 150 Valsartan and Hydrochlorothiazide Analyzed with HPLC pdf 0.5 Mb Download File