Simple Separation of API from Melamine and Cyanoguanidine
A simple Method was developed for the analysis of the widely prescribed anti-diabetic drug Metformin Hydrochloride and for determination of two impurities, Cyanoguanidine and Melamine, in tablet formulations.
In addition, to Accurate Mass all three compounds, the Peaks were confirmed by injections of standards. The USP specified impurity limits are not more than 0.02% and 0.01% for Cyanoguanidine and Melamine respectively.
The precision of this Method was evaluated by calculating %RSD of the Peak areas of five injections (see Figure B). The obtained value was 0.2%.
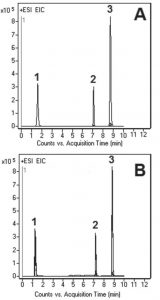
 Peaks:
Peaks:
1. Cyanoguanidine 85.0509 m/z (M+H)+
2. Melamine 127.0727 m/z (M+H)+
3. Metformin 130.1087 m/z (M+H)+
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-15P-2
Dimensions: 2.1 x 150mm
Mobile Phase:
—A: 50% Isopropanol / 50% DI Water / 0.1% Acetic Acid
—B: Acetonitrile / 0.1% Acetic Acid
Gradient:
| Time (minutes) | %B |
| 0 | 100 |
| 2 | 100 |
| 5 | 20 |
| 9 | 20 |
| 10 | 100 |
Post Time: 5 minutes
Injection vol.: 1µL
Flow rate: 0.4mL / minute
Detection: ESI – POS – Agilent 6210 MSD TOF Mass Spectrometer
Note: k’ of Cyanoguanidine for this Method was found to be over twice that of some Reversed Phase Methods studies that used ordinary Cyano Columns and an isocratic Mobile Phase 95% DI Water / 5% Acetonitrile. A variety of Mobile Phase additives were investigated, including 0.1% Formic Acid, 0.1% Trifluoroacetic Acid (TFA), 0.1% Trifluoroacetic Acid (TFA) +1g / L Sodium Octyl Sulfate, and 10mM Ammonium Acetate. The Reversed Phase Methods did not produce k’ > 0.3.
Attachment
No 146 Impurities Determination in Metformin HCL Formulation pdf 0.3 Mb Download File


