Robust Separation of API from USP Internal Standard
The USP Assay Method for Alprazolam uses a bare Silica Column and a complex Mobile Phase consisting of Acetonitrile, Chloroform, Butyl Alcohol, and Acetic Acid. In this Method, a simple LCMS compatible Mobile Phase is used and produces excellent Peak shapes for both the API and its USP internal standard.
Furthermore, a resolution of 4.3 was obtained between the two Peaks, which meets the USP system suitability of Rs≥2.0. Two impurity Peaks are also observed, which further illustrates the resolution capabilities of the analysis. This Method illustrates how the MS-compatible HPLC-UV Methods described in various application notes can be successfully adapted for LCMS.
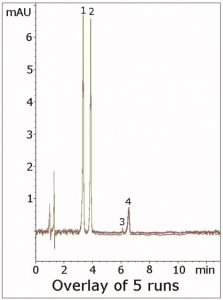
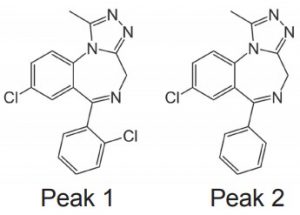 Peaks:
Peaks:
1. Triazolam (internal standard)
2. Alprazolam (API)
3, 4. Impurities
Method Conditions
Column: Cogent Diamond Hydride™, 4µm, 100Å
Catalog No.: 70000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 95 |
| 1 | 95 |
| 6 | 50 |
| 7 | 95 |
Post Time: 3 minutes
Injection vol.: 1µL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation:
—Tablet: A 0.25mg strength generic Xanax® tablet was ground and added to a 10mL volumetric flask. After diluting with 50% Solvent A / 50% Solvent B, it was sonicated for 10 minutes. A portion was filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech Corp.).
—Internal Standard: 1mg / mL Triazolam in Methanol diluent.
—Working Solutions: 20µL of the internal standard and 980µL of the tablet extract were mixed. Peak identities were confirmed by individual solutions of the tablet extract and the internal standard.
t0: 0.9 minutes
Note: Alprazolam is a member of the benzo-diazepine class of compounds, prescribed to treat a variety of anxiety-related conditions.
Attachment
No 183 Alprazolam Xanax Analyzed with HPLC pdf 0.6 Mb Download File


