USP Method with an SDS Resistant Column
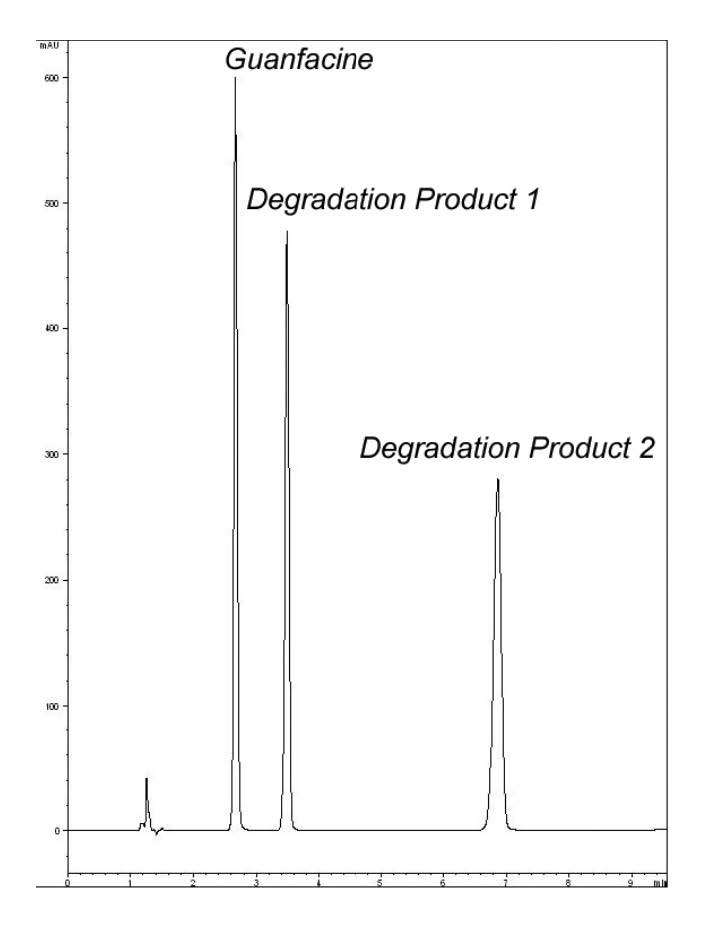
The US Pharmacopeia (USP) lists several known degradation products for Guanfacine. The Figure below shows a Separation of a degraded Guanfacine Drug Substance. This Method produces very Efficient results but what is important to note is that the Column used is extremely stable under the very aggressive Mobile Phase Conditions that are needed.
Using Columns based on Silica Hydride instead of ordinary Silica offers not only Stability but also great compatibility with Sodium Dodecyl Sulfate (SDS) or other ion pair reagents providing very long Column lifetime as well as more stable results.
Peaks:
1. Guanfacine
2. Primary Degradant 1
3. Primary Degradant 2
Method Conditions
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40008-15P
Dimensions: 4.6 x 150mm
Mobile Phase: 30:70 Acetonitrile / DI Water (with concentrated Phosphoric Acid (1mL / L), 1g / L SDS).
Temperature: 25°C
Injection vol.: 20μL
Flow rate: 1.5mL / minute
Detection: UV @ 220nm
Notes: Guanfacine can be used to control high blood pressure by reducing the heart rate and relaxing blood vessels but also is prescribed for ADD and ADHD and sometimes for Post-Traumatic Stress Syndrome. Guanfacine has been known to reduce nightmares and flashbacks.
Attachment
No 72 Guanfacine HCL and Degradants Analyzed with HPLC pdf 0.2 Mb Download File