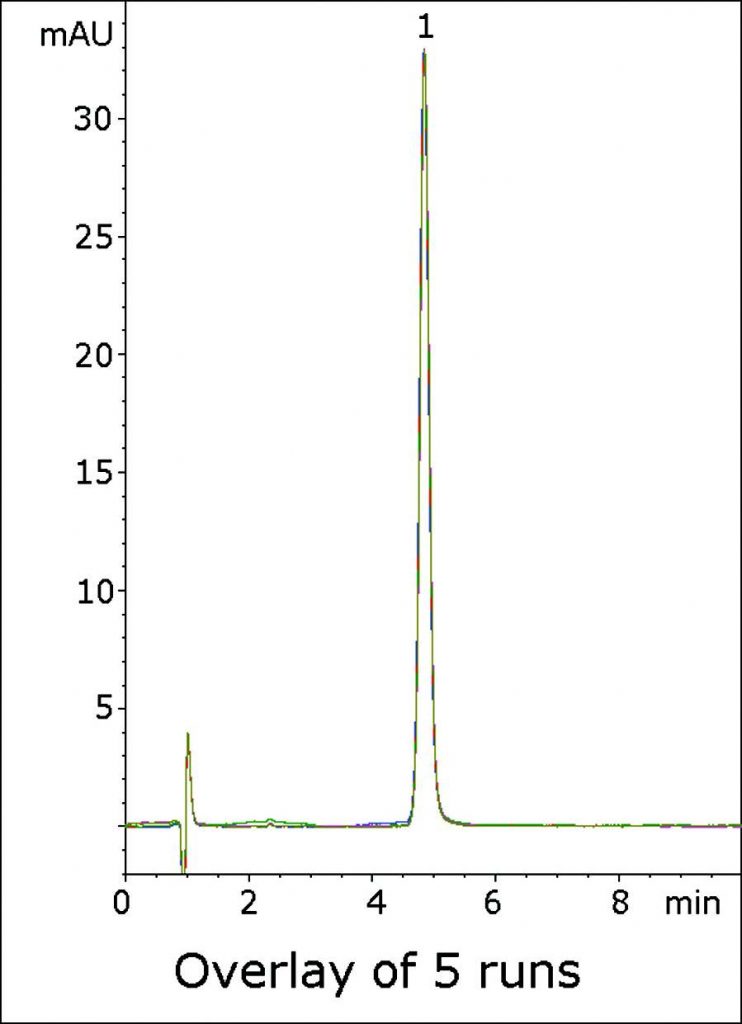
Excellent Peak Shape and Precision for a Benzodiazepine Compound Valium®
This Isocratic Method shows how using 0.1% Trifluoroacetic Acid (TFA) in the Mobile Phase can produce a Peak with excellent Efficiency and Symmetry. In the USP Assay Method, System Suitability requires that a tailing factor of not more than 2.0 be obtained for the API, and this Method produces a Peak that is well within the Specification.
An overlay of Five consecutive chromatograms is shown in the Figure to illustrate the Precision and Robustness of the Method.
Peak:
Diazepam
Method Conditions
Column: Cogent Bidentate C8™, 4μm, 100Å
Catalog No.: 40008-75P
Dimensions: 4.6 x 75mm
Mobile Phase: 70:30 DI Water / Acetonitrile with 0.1% Trifluoroacetic Acid (TFA) v/v
Injection vol.: 10μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: 10mg of a ground Valium® tablet was added to a 10mL volumetric flask containing a portion of a 50:50 Acetonitrile / DI Water diluent. The flask was sonicated 10 minutes and diluted to mark. A portion was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Diazepam is a benzodiazepine used to treat conditions such as anxiety, muscle spasms, insomnia, seizures, and to control agitation caused by alcohol withdrawal. It is marketed as Valium® by Hoffmann-La Roche, although generic versions are currently available.
Attachment
No 190 Diazepam Tablet Analyzed with HPLC pdf 0.3 Mb