Separation of Estriol, Estradiol, & Progesterone
This gradient method features a separation of the three components of a hormone replacement formulation. Figure A shows a five run overlay of the formulation extract injections. Figure B shows a zoomed-in view so that the Estriol and Estradiol Peaks, which are present in much lower concentration than Progesterone, can be seen clearly. Figure B also shows separation of an impurity from the Progesterone Peak.

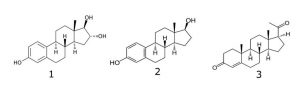
Peak:
1. Estriol
2. Estradiol
3. Progesterone
Method Conditions
Column: Cogent UDC Cholesterol™, 4μm, 100Å
Catalog No.: 69069-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase:
—A: DI Water / 0.1% Formic Acid (v/v)
—B: Acetonitrile / 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 33 |
| 2 | 33 |
| 11 | 65 |
| 12 | 33 |
Post Time: 3 minutes
Flow rate: 1.0 mL / minute
Detection: UV @ 210nm
Injection vol.: 1μL
Sample Preparation: The contents of a capsule containing 0.124 mg Estradiol, 1.001 mg Estriol, and 50 mg Progesterone were added to a 25 mL volumetric flask. The flask was diluted to mark with solvent B and sonicated 10 minutes. Then A portion was filtered with a 0.45µm Nylon Syringe Filter (MicroSolv Tech. Corp.). Peak identities were confirmed by individual standards of 0.1 mg / mL in a Solvent B diluent.
Attachment
No 175 Hormone Replacement Capsule Analysis pdf 0.4 Mb Download File


