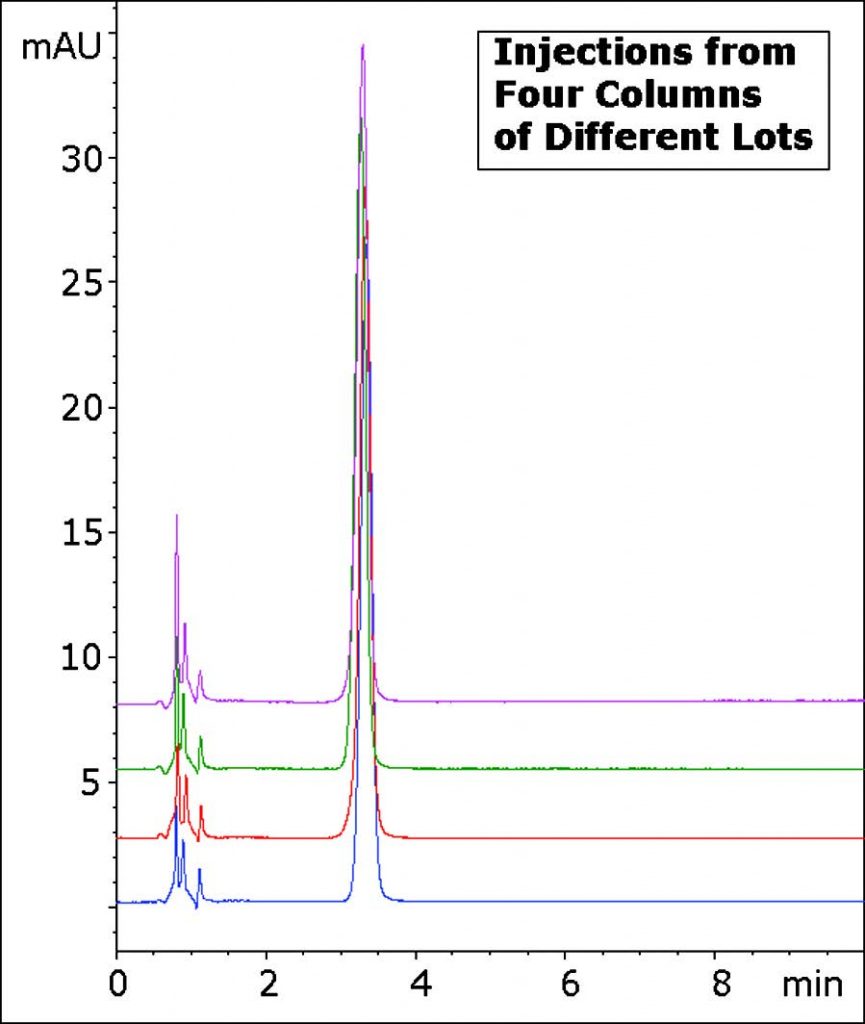
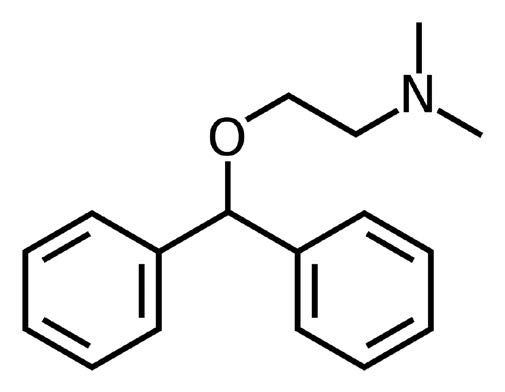
Assay Method for Diphenhydramine HCL without Ion Pairing Agents
Diphenhydramine has a tertiary amine functional group that can produce tailing with conventional Columns in Reversed Phase HPLC. The USP Method features a Triethylamine additive in the Mobile Phase for this reason. These additives often take a significant time to completely load onto the Column and therefore adversely affect throughput and Robustness.
The Method in this Application Note produces an excellent Peak Shape using only Ammonium Acetate as a Mobile Phase Additive. In addition, the Reproducibility and Robustness of this Method is demonstrated through the overlay of four Chromatograms with Columns from four different batches.
Peak:
Diphenhydramine
Method Conditions
Column: Cogent Silica-C™, 4μm, 100Å
Catalog No.: 40000-7.5P
Dimensions: 4.6 x 75mm
Mobile Phase: 50:50 DI Water / Acetonitrile with 5mM Ammonium Acetate
Injection vol.: 2μL
Flow rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: 25mg strength Benadryl® (Diphenhydramine) capsule was opened and placed in a 10mL volumetric flask with a portion of the Mobile Phase as diluent. It was sonicated 10 minutes and diluted to mark. Then a portion was filtered with a 0.45μm Nylon Syringe Filter (MicroSolv Tech Corp.).
t0: 0.9 minutes
Note: Diphenhydramine is a first generation antihistamine used primarily to treat allergies. It also has a significant sedative property, which is sometimes an undesirable side effect of its intended use. However, it is used in many formulations as a sleep aid as well.
Attachment
No 194 Diphenhydramine HCL Capsule Analyzed with HPLC.pdf 0.4 Mb Download File