Click HERE for Column Ordering Information.
A rapid, sensitive, and Reproducible Method has been developed for analysis of Ticagrelor. The data below, (overlay of 5 chromatograms ) illustrates how the compound can be adequately Retained and detected using a simple Gradient in Reversed Phase HPLC. The Method demonstrates good Peak Shape and run-to-run Precision with RSD values less than 0.3%.
A Phenyl ring in the Column Stationary Phase provides beneficial π-π Interaction with the Analyte making possible the use of a very simple, Mass Spec friendly Mobile Phase with Formic Acid as an additive.
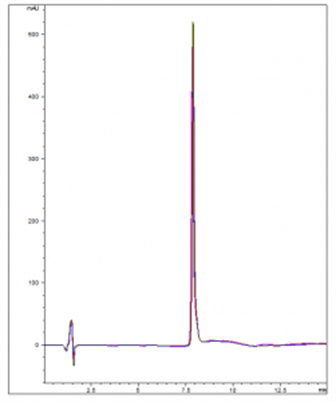
5 Injections of Ticagrelor Overlay
Method Conditions
Column: Cogent Phenyl Hydride™, 4μm, 100Å
Catalog No.: 69020-10P-2
Dimensions: 2.1mm x 100mm
Mobile Phase:
--A: DI Water with 0.1% Formic Acid (v/v)
--B: Acetonitrile with 0.1% Formic Acid (v/v)
Gradient:
| Time (minutes) | %B |
| 0 | 40 |
| 1 | 40 |
| 5 | 85 |
| 6 | 85 |
| 7 | 40 |
| 10 | 40 |
Flow rate: 0.2mL / minute
Detection: UV @ 254nm
Sample Preparation: 2.0mg / mL Ticagrelor standard solution in Methanol.
Notes: Ticagrelor is an oral prescription indicated for treatment of acute ischemic stroke, coronary artery disease and acute coronary syndrome.

