Lower retention is observed when functional group in a compound is ionized - Tips & Suggestions
November 6, 2013
/
/
/
/
/
/
/
/
/
/
I am analyzing S-adenosyl methionine (SAMe) with the Cogent Diamond Hydride™ column with an Aqueous Normal Phase ANP HPLC method.
I used a gradient with a formic acid additive and then tried an ammonium acetate additive. With ammonium acetate, I got lower retention than with formic acid using otherwise identical method conditions.
I was expecting greater retention with ammonium acetate since the carboxyl group would be ionized at this pH but not with formic acid. I expected that the compound would be more polar if it were ionized and therefore would retain longer. What happened?
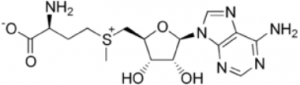
Suggestion: The compound also has a permanent positive charge on the sulfur so if the carboxyl is negatively charged, these charges may cancel out. Hence it may be less polar under these conditions and retain less by ANP. Try methods with formic acid, acetic acid, TFA, or other acid additive.
Click HERE for Diamond Hydride™ HPLC column ordering information

I used a gradient with a formic acid additive and then tried an ammonium acetate additive. With ammonium acetate, I got lower retention than with formic acid using otherwise identical method conditions.
I was expecting greater retention with ammonium acetate since the carboxyl group would be ionized at this pH but not with formic acid. I expected that the compound would be more polar if it were ionized and therefore would retain longer. What happened?

Suggestion: The compound also has a permanent positive charge on the sulfur so if the carboxyl is negatively charged, these charges may cancel out. Hence it may be less polar under these conditions and retain less by ANP. Try methods with formic acid, acetic acid, TFA, or other acid additive.
Click HERE for Diamond Hydride™ HPLC column ordering information

