Molnupiravir and Favipiravir Analyzed with HPLC - AppNote
October 12, 2021
/
/
/
/
/
Oral Anti-Viral Medications - Easy Reversed Phase Method
Molnupiravir and Favipiravir, new anti-viral drugs were analyzed by HPLC using a simple Mobile Phase. As shown in the 10 injection overlay in the chromatogram below, the Separation, Peak Shapes and Repeatability are very good (%RSD ≤ 0.2).
Column: Cogent RP C18™, 5µm, 100Å
Catalog No.: 68518-15P
Dimensions: 4.6 x 150mm
Mobile Phase: (75:25) DI Water / Acetonitrile with 0.1% Formic Acid
Injection Volume: 1µL
Flow Rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: Molnupiravir and Favipiravir are dissolved at a concentration of 0.5mg / mL in (50:50) DI Water / Acetonitrile
Note: Molnupiravir is an oral antiviral drug that was developed for the treatment of influenza. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication. Favipiravir is effective against a wide range of types and subtypes of influenza viruses, including strains resistant to existing anti-influenza drugs. Of note is that favipiravir shows anti-viral activities against other RNA viruses such as arenaviruses, bunyaviruses and filoviruses, all of which are known to cause fatal hemorrhagic fever.

Molnupiravir and Favipiravir, new anti-viral drugs were analyzed by HPLC using a simple Mobile Phase. As shown in the 10 injection overlay in the chromatogram below, the Separation, Peak Shapes and Repeatability are very good (%RSD ≤ 0.2).
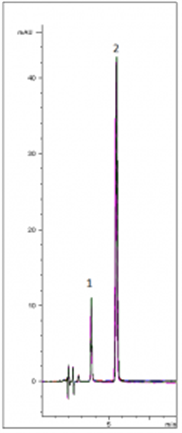
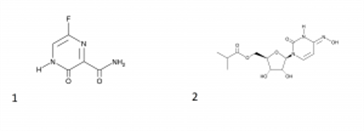
Peaks:
1. Favipiravir
2. Molnupiravir
Column: Cogent RP C18™, 5µm, 100Å
Catalog No.: 68518-15P
Dimensions: 4.6 x 150mm
Mobile Phase: (75:25) DI Water / Acetonitrile with 0.1% Formic Acid
Injection Volume: 1µL
Flow Rate: 1.0mL / minute
Detection: UV @ 254nm
Sample Preparation: Molnupiravir and Favipiravir are dissolved at a concentration of 0.5mg / mL in (50:50) DI Water / Acetonitrile
Note: Molnupiravir is an oral antiviral drug that was developed for the treatment of influenza. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication. Favipiravir is effective against a wide range of types and subtypes of influenza viruses, including strains resistant to existing anti-influenza drugs. Of note is that favipiravir shows anti-viral activities against other RNA viruses such as arenaviruses, bunyaviruses and filoviruses, all of which are known to cause fatal hemorrhagic fever.

