How can I get separation of tartaric and malic acid in a complex mixture? - FAQ.
April 14, 2020
/
/
/
/
/
/
Suppose you have a sample containing lactic acid, glycolic acid, formic acid, L-tartaric acid, DL-malic acid, and succinic acid. The Cogent Diamond Hydride™ HPLC column will be an excellent column choice for this separation. In the mobile phase that you will use, you will want to keep the acids ionized so that they will be more strongly retained on the column utilizing Aqueous Normal Phase (ANP) HPLC.
To do this, use a 10mM ammonium acetate additive in solvent A and 90/10 acetonitrile/10mM ammonium acetate in solvent B. For detection, use negative ion mode in LC-MS and look for the [M-H]- peaks in the EIC. For the gradient, start with the following:
Time (min) %B
0.0 90
6.0 30
9.0 30
10.0 90
You may find that tartaric and malic acid co-elute under these conditions (peaks 4 and 5):

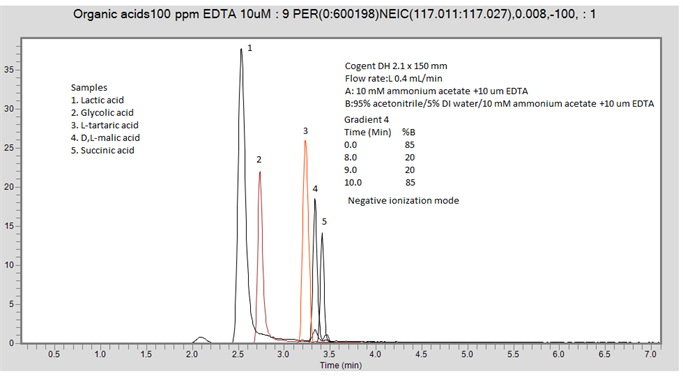
In this case, you can try a different gradient to get separation of peaks 4 and 5:
Time (min) %B
0.0 85
8.0 20
9.0 20
10.0 85
Click HERE for Cogent Diamond Hydride HPLC Column Ordering Information.

To do this, use a 10mM ammonium acetate additive in solvent A and 90/10 acetonitrile/10mM ammonium acetate in solvent B. For detection, use negative ion mode in LC-MS and look for the [M-H]- peaks in the EIC. For the gradient, start with the following:
Time (min) %B
0.0 90
6.0 30
9.0 30
10.0 90
You may find that tartaric and malic acid co-elute under these conditions (peaks 4 and 5):

In this case, you can try a different gradient to get separation of peaks 4 and 5:
Time (min) %B
0.0 85
8.0 20
9.0 20
10.0 85

Click HERE for Cogent Diamond Hydride HPLC Column Ordering Information.

