Amide vs. Amine Functional Groups in HPLC Applications -
Update 28-MAY-2025
It is important to distinguish between amide and amine functional groups, particularly in the context of HPLC method development and stationary phase selection.
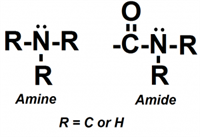
While both groups contain nitrogen, the amide group includes a carbonyl (C=O) adjacent to the nitrogen, which significantly alters its chemical behavior. This structural difference results in key distinctions:
-
Ionizability:
- Amines are basic and ionizable, often carrying a positive charge under acidic or neutral conditions.
- Amides, in contrast, are non-ionizable under typical chromatographic conditions due to resonance stabilization of the nitrogen lone pair with the adjacent carbonyl.
-
Reactivity:
- Amines are nucleophilic and can readily react with electrophilic species such as aldehydes, potentially forming Schiff bases or other adducts.
- Amides are significantly less reactive, making them more stable in chemically diverse sample matrices.
These differences have practical implications in chromatography. For example, if an amine group on a stationary phase reacts with a sample component (e.g., an aldehyde), the resulting covalent bond alters the surface chemistry of the column. This irreversible modification can lead to loss of retention characteristics and is referred to as stationary phase deactivation.
Why Choose Amide-Based Columns?
Amide-functionalized stationary phases offer several advantages:
- Reduced chemical reactivity, minimizing unwanted interactions with reactive analytes.
- Improved reproducibility in gradient and complex sample analyses.
- Enhanced stability in the presence of electrophilic compounds.
For analytes prone to reacting with amine groups, or when working with reactive sample matrices, amide columns provide a more inert and reliable alternative, helping preserve column performance and analytical integrity.