What is 0.1 % w/v ammonium acetate in terms of molarity - FAQ
April 14, 2020
/
/
/
/
Percentage given as w/v means weight over volume. This is typically used for solids dissolved in a liquid.
Ammonium acetate is a solid and is often used in HPLC mobile phases. Suppose you have a 0.1 % (w/v) ammonium acetate solution but your SOP requires you to state the concentration in mM. How do you convert? You will need the molecular weight of the solute (77.1 g/mol in this case.)
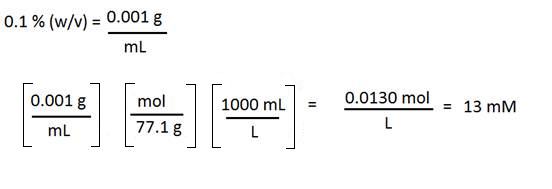
1. Convert the percent to grams per mL
2. Change grams to mol (use the molecular weight)
3. Change mL to L
4. You will get mol per liter, or M. This may be expressed as mM by multiplying by 1000.
Ammonium acetate is a solid and is often used in HPLC mobile phases. Suppose you have a 0.1 % (w/v) ammonium acetate solution but your SOP requires you to state the concentration in mM. How do you convert? You will need the molecular weight of the solute (77.1 g/mol in this case.)
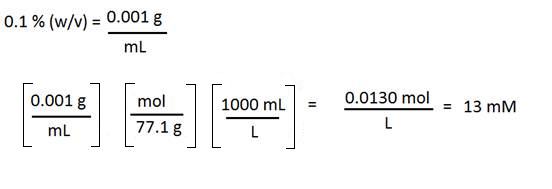
1. Convert the percent to grams per mL
2. Change grams to mol (use the molecular weight)
3. Change mL to L
4. You will get mol per liter, or M. This may be expressed as mM by multiplying by 1000.
