Sitagliptin phosphate USP assay with HPLC – AppNote INTERNAL ONLY
June 23, 2022
/
/
/
/
/
/
/
CONFIDENTIAL – INTERNAL ONLY. Click here for public AppNote.
Alternate L10 column used for a similar USP assay method of sitagliptin phosphate
USP sitagliptin phosphate is easily performed and the 10 injection overlay below demonstrates run to run consistency and great peak symmetry. The peak efficiency is very good and peak tailing guidelines are easily met with RSD values of less than 1.0%.
This demonstrates a good alternate column for your sitagliptin phosphate USP method.

Column: Cogent NP Cyano™, 5um, 100Å
Catalog No.: 82534-15P
Dimensions: 4.6mm x 150mm
Mobile Phase:
--Buffer: 1.36 g/L of Monobasic Potassium Phosphate, adjusted with Phosphoric Acid to a pH of 2.0
--Mobile phase: Acetonitrile and Buffer (15:85)
--Dilute phosphoric acid: Transfer 1 mL of phosphoric acid to a 1-L volumetric flask and dilute with water to volume.
--Diluent: Acetonitrile and Dilute Phosphoric Acid (5:95)
Injection vol.: 20μL
Flow rate: 1.0mL / minute
Detection: UV @ 205nm
Column temperature: 30°C
Sample Preparation: 0.1 mg/mL of Sitagliptin Phosphate in Diluent
Peak Tailing: NMT 1.5%
%RSD of 10 injections: ‹1.0%
Note 1: For the full method conditions and details, consult the official United States Pharmacopeia–National Formulary (USP–NF.)
Note 2: Sitagliptin is an oral dipeptidyl peptidase-4 (DPP-4) inhibitor used in conjunction with diet and exercise to improve glycemic control in patients with type 2 Diabetes

Alternate L10 column used for a similar USP assay method of sitagliptin phosphate
USP sitagliptin phosphate is easily performed and the 10 injection overlay below demonstrates run to run consistency and great peak symmetry. The peak efficiency is very good and peak tailing guidelines are easily met with RSD values of less than 1.0%.
This demonstrates a good alternate column for your sitagliptin phosphate USP method.

Peak:
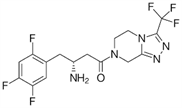
Sitagliptin Phosphate
Method Conditions:Column: Cogent NP Cyano™, 5um, 100Å
Catalog No.: 82534-15P
Dimensions: 4.6mm x 150mm
Mobile Phase:
--Buffer: 1.36 g/L of Monobasic Potassium Phosphate, adjusted with Phosphoric Acid to a pH of 2.0
--Mobile phase: Acetonitrile and Buffer (15:85)
--Dilute phosphoric acid: Transfer 1 mL of phosphoric acid to a 1-L volumetric flask and dilute with water to volume.
--Diluent: Acetonitrile and Dilute Phosphoric Acid (5:95)
Injection vol.: 20μL
Flow rate: 1.0mL / minute
Detection: UV @ 205nm
Column temperature: 30°C
Sample Preparation: 0.1 mg/mL of Sitagliptin Phosphate in Diluent
Peak Tailing: NMT 1.5%
%RSD of 10 injections: ‹1.0%
Note 1: For the full method conditions and details, consult the official United States Pharmacopeia–National Formulary (USP–NF.)
Note 2: Sitagliptin is an oral dipeptidyl peptidase-4 (DPP-4) inhibitor used in conjunction with diet and exercise to improve glycemic control in patients with type 2 Diabetes

