Flumazenil USP assay with HPLC - INTERNAL ONLY
October 27, 2022
/
/
/
/
/
/
/
CONFIDENTIAL – INTERNAL ONLY. Click here for public AppNote.
Alternate L1 column used for a similar USP assay method of Flumazenil
This Similar USP Flumazenil is easily performed and the 10 injection overlay below demonstrates run to run consistency and great peak symmetry. The peak efficiency is very good and peak tailing guidelines are easily met with RSD values of less than 1.0%.
This demonstrates a good alternate column for your Flumazenil USP method.
Column: Cogent RP C18™, 5um, 100Å
Catalog No.: 68518-15P
Dimensions: 4.6mm x 150mm
Solution A: 800 mL of water, adjusted with phosphoric acid to a pH of 2.0 ± 0.05
Mobile phase: Methanol, tetrahydrofuran, and Solution A (13:7:80)
Flow rate: 1.0mL / minute
Detection: UV @ 230nm
Sample Preparation: 1.0 mg/mL of Flumazenil in Mobile phase
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Note 1: For the full method conditions and details, consult the official United States Pharmacopeia–National Formulary (USP–NF.)
Note 2: Flumazenil is a GABA receptor antagonist and can treat drowsiness caused by sedatives following surgery or drug overdose.
.

Alternate L1 column used for a similar USP assay method of Flumazenil
This Similar USP Flumazenil is easily performed and the 10 injection overlay below demonstrates run to run consistency and great peak symmetry. The peak efficiency is very good and peak tailing guidelines are easily met with RSD values of less than 1.0%.
This demonstrates a good alternate column for your Flumazenil USP method.
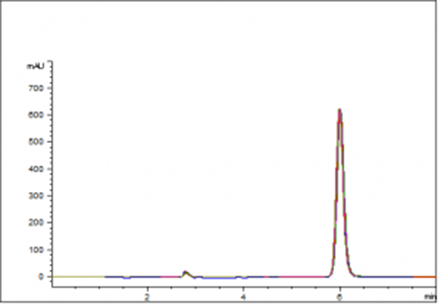
Peak:
Flumazenil
Column: Cogent RP C18™, 5um, 100Å
Catalog No.: 68518-15P
Dimensions: 4.6mm x 150mm
Solution A: 800 mL of water, adjusted with phosphoric acid to a pH of 2.0 ± 0.05
Mobile phase: Methanol, tetrahydrofuran, and Solution A (13:7:80)
Flow rate: 1.0mL / minute
Detection: UV @ 230nm
Sample Preparation: 1.0 mg/mL of Flumazenil in Mobile phase
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Note 1: For the full method conditions and details, consult the official United States Pharmacopeia–National Formulary (USP–NF.)
Note 2: Flumazenil is a GABA receptor antagonist and can treat drowsiness caused by sedatives following surgery or drug overdose.
.

